 | |
 | |
| Names | |
|---|---|
| IUPAC name 1,1,1,3,3,3-hexafluoro- | |
| Other names perfluoroacetone acetone hexafluoride perfluoro-2-propanone | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.616 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2420 |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C3F6O | |
| Molar mass | 166.02 g/mol |
| Appearance | Colorless gas |
| Odor | musty[1] |
| Density | 1.32 g/ml, liquid |
| Melting point | −129 °C (144 K) |
| Boiling point | −28 °C (245 K) |
| Reacts with water | |
| Vapor pressure | 5.8 atm (20 °C)[1] |
| Hazards | |
| Main hazards | Toxic (T), Corrosive (C) |
| GHS pictograms |      |
| GHS Signal word | Danger |
GHS hazard statements | H280, H301, H310, H311, H314, H315, H318, H330, H360, H370, H372 |
GHS precautionary statements | P201, P202, P260, P262, P264, P270, P271, P280, P281, P284, P301+310, P301+330+331, P302+350, P302+352, P303+361+353, P304+340, P305+351+338, P307+311, P308+313, P310, P312, P314, P320, P321, P322 |
| NFPA 704 (fire diamond) | |
| Flash point | Nonflammable[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | none[1] |
REL (Recommended) | TWA 0.1 ppm (0.7 mg/m3) [skin][1] |
IDLH (Immediate danger) | N.D.[1] |
| Related compounds | |
Related ketones; organofluorides | Acetone; Hexafluoro-2-propanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
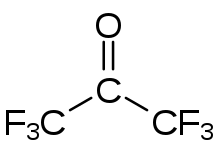
Hexafluoroacetone (HFA) is a chemical compound with the formula (CF3)2CO. It is structurally similar to acetone; however, its reactivity is markedly different. It a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour.[2] The most common form of this substance is hexafluoroacetone sesquihydrate (1.5 H2O), which is a hemihydrate of hexafluoropropane-2,2-diol (F
3C)
2C(OH)
2, a geminal diol .
Synthesis
The industrial route to HFA involves treatment of hexachloroacetone with HF:[3]
- (CCl3)2CO + 6 HF → (CF3)2CO + 6 HCl
Hexafluoropropylene oxide rearranges to give HFA.
In the laboratory, HFA can be prepared in a two step process from perfluoropropene. In the first step KF catalyzes the reaction of the alkene with elemental sulfur to give the 1,3-dithietane [(CF3)2CS]2. This species is then oxidized by iodate to give (CF3)2CO.[4]
Uses
Hexafluoroacetone is used in the production of hexafluoroisopropanol:
- (CF3)2CO + H2 → (CF3)2CHOH
It is also used as a precursor to hexafluoroisobutylene,[3] a monomer used in polymer chemistry, and as a building block in the synthesis of midaflur, bisphenol AF, 4,4′-(hexafluoroisopropylidene)diphthalic anhydride, and alitame.
Reactivity
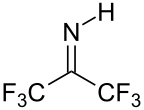
Hexafluoroacetone is an electrophile. Nucleophiles attack at the carbonyl carbon. In water, hexafluoroacetone predominantly exists as the hydrate. The equilibrium constant (Keq) for the formation of this geminal diol is 106 M−1. The analogous equilibrium for acetone is an unfavorable 10−3 M−1.[5] Hexafluoroacetone-hydrates are acidic. In an analogous reaction, ammonia adds to hexafluoroacetone to give the hemiaminal (CF3)2C(OH)(NH2) which can be dehydrated with phosphoryl chloride to give the imine (CF3)2CNH.[6]
See also
- Bromoacetone
- Chloroacetone
- Fluoroacetone
- Trifluoroacetone
- Novec 1230
- Hexafluorothioacetone
References
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0319". National Institute for Occupational Safety and Health (NIOSH).
- ^ CDC - NIOSH Pocket Guide to Chemical Hazards
- ^ a b Günter Siegemund; Werner Schwertfeger; Andrew Feiring; Bruce Smart; Fred Behr; Herward Vogel; Blaine McKusick (2002). "Fluorine Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- ^ Van Der Puy, M.; Anello, L. G. (1990). "Hexafluoroacetone". Organic Syntheses.; Collective Volume, 7, p. 251
- ^ Lemal, David M. (2004). "Perspective on Fluorocarbon Chemistry". The Journal of Organic Chemistry. 69 (1): 1–11. doi:10.1021/jo0302556. PMID 14703372.
- ^ W. J. Middleton; H. D. Carlson (1970). "Hexafluoroacetone imine". Org. Syntheses. 50: 81–3. doi:10.15227/orgsyn.050.0081..