 | |
 | |
| Names | |
|---|---|
| IUPAC name 1,1,1,3,3,3-Hexafluoro-propan-2-ol | |
| Other names Hexafluoroisopropanol, Hexafluoroisopropyl alcohol, HFIP | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.011.873 |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C3H2F6O | |
| Molar mass | 168.038 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.596 g/mL |
| Melting point | −3.3 °C (26.1 °F; 269.8 K) |
| Boiling point | 58.2 °C (136.8 °F; 331.3 K) |
| Miscible | |
| Vapor pressure | 16 kPa at 20 °C |
| Viscosity | 1.65 cP at 20 °C |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements | H314, H361fd, H373 |
GHS precautionary statements | P201, P280, P303+361+353, P305+351+338+310, P308+313 |
| NFPA 704 (fire diamond) | |
| Flash point | > 100 °C (212 °F; 373 K) |
| Related compounds | |
Related Organofluorides; alcohols | Hexafluoroacetone; Isopropyl alcohol, 2,2,2-Trifluoroethanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
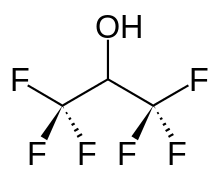
Hexafluoroisopropanol, commonly abbreviated HFIP, is the organic compound with the formula (CF3)2CHOH. This fluoroalcohol finds use as solvent and synthetic intermediate. It appears as a colorless, volatile liquid that is characterized by a strong, pungent odor. As a solvent hexafluoro-2-propanol is polar and exhibits strong hydrogen bonding properties enabling it to dissolve substances that serve as hydrogen-bond acceptors, such as amides and ethers. (CF3)2CHOH is classified as a hard Lewis acid and its acceptor properties are discussed in the ECW model. Its relative acceptor strength toward a series of bases, versus other Lewis acids, can be illustrated by C-B plots.[1][2] Hexafluoro-2-propanol is transparent to UV light with high density, low viscosity and low refractive index.
Production and uses
Hexafluoro-propan-2-ol is prepared from hexafluoropropylene through hexafluoroacetone, which is then hydrogenated.[3]
- (CF3)2CO + H2 → (CF3)2CHOH
Hexafluoro-propan-2-ol is a speciality solvent for some polar polymers and organic synthesis.[4][5] It is especially effective for solubilizing a wide range of polymers, including those that are not soluble in the most common organic solvents, such as: polyamides, polyacrylonitriles, polyacetals, polyesters (e.g. polyglycolide), and polyketones. It has also found use in biochemistry to solubilize peptides and to monomerize β-sheet protein aggregates. Because of its acidity (pKa = 9.3), it can be used as acid in volatile buffers for ion pair HPLC - mass spectrometry of nucleic acids.[6]
Medicine
It is both the precursor and the chief metabolite of the inhalation anesthetic sevoflurane.
Safety
Hexafluoro-2-propanol is a volatile, corrosive liquid that can cause severe burns and respiratory problems. With prolonged or repeated exposure, HFIP is likely to impair fertility as well as harm the unborn child.[7]
Environment
The release of hexafluoroisopropanol into the environment must be avoided. HFIP belongs to the per- and polyfluoroalkyl substances.[8] These so-called PFAS, also known as forever chemicals, are considered non-degradable substances in nature due to their fluorination. They are therefore classified as persistent organic pollutants. Due to its good solubility in water, hexafluoroisopropanol is also highly mobile. Thus, hexafluoroisopropanol counts as a vPvM/PMT substance (very persistent, mobile substances that may have toxic potential).[9] As a volatile and fluorinated substance, hexafluoroisopropanol increases global warming. It has a global warming potential (GWP) of 195, making it 195 times more harmful to the atmosphere than CO2.[10]
References
- ^ Laurence, C. and Gal, J-F. Lewis Basicity and Affinity Scales, Data and Measurement, (Wiley 2010) pp 50-51 IBSN 978-0-470-74957-9
- ^ Cramer, R. E., and Bopp, T. T. (1977) Great E and C plot. Graphical display of the enthalpies of adduct formation for Lewis acids and bases. Journal of Chemical Education 54 612-613, Improved E&C parameters are listed in ECW model. The plots shown in this paper used older parameters.
- ^ Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” in Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, 2007. doi:10.1002/14356007.a11_349
- ^ Bégué, J.-P.; Bonnet-Delpon, D.; Crousse, B. (2004). "Fluorinated Alcohols: A New Medium for Selective and Clean Reaction". Synlett (1): 18–29. doi:10.1055/s-2003-44973.
- ^ Shuklov, Ivan A.; Dubrovina, Natalia V.; Börner, Armin (2007). "Fluorinated Alcohols as Solvents, Cosolvents and Additives in Homogeneous Catalysis". Synthesis. 2007 (19): 2925–2943. doi:10.1055/s-2007-983902.
- ^ Apffel, A.; Chakel, J.A.; Fischer, S.; Lichtenwalter, K.; Hancock, W.S. (1997). "Analysis of oligonucleotides by HPLC-electrospray ionization mass spectrometry". Anal. Chem. 69: 1320–1325. doi:10.1021/ac960916h. PMID 21639339.
- ^ "HFIP MSDS". Sigma Aldrich. Retrieved 7 January 2021.
- ^ "PFAS structures in DSSTox". CompTox. Retrieved 7 January 2021.
- ^ Arp, Hans Peter (November 2019). REACH: Improvement of guidance and methods for the identification and assessment of PMT/vPvM substances (PDF). Umweltbundesamt. Retrieved 7 January 2021.
- ^ "MANDATORY GREENHOUSE GAS REPORTING" (PDF). epa.gov. Federal Register. Retrieved 7 January 2021.
Sources
- Radlick, Phillip C (1982-02-02). "Methods of synthesizing hexafluoroisopropanol from impure mixtures and synthesis of a fluoromethyl ether therefrom". United States Patent 4,314,087. Retrieved 2006-10-18.
- Cheminal, Bernard; H. Mathais; M. Thomarat (1987-03-03). "Process for the synthesis of 2,2,2-trifluoroethanol and 1,1,1,3,3,3-hexafluoroisopropanol". United States Patent 4,647,706. Retrieved 2006-10-18.
