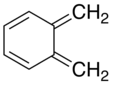
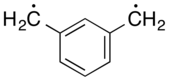
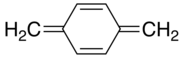
Xylylene comprises two isomeric organic compounds with the formula C6H4(CH2)2. These compounds are related to the corresponding quinones by replacement of the oxygen atoms by CH2 groups. ortho- and para-xylylene are best known, although neither is stable in solid or liquid form. The meta form is a diradical. Certain substituted derivatives of xylylenes are however highly stable, an example being tetracyanoquinodimethane.
p-Xylylene
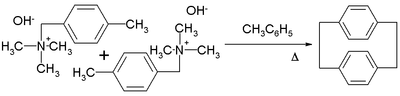
p-Xylylene forms upon pyrolysis of p-xylene or, more readily, the α-substituted derivatives. p-Xylylene dimerizes with moderate efficiency to give p-cyclophane:[1]
Further heating of the p-cyclophane gives poly(para-xylylene).
o-Xylylene
Reaction of tetrabromo-o-xylene (C6H4(CHBr2)2) with sodium iodide affords α,α'-dibromo-o-xylylene, which can be trapped with dienophiles to give naphthylene derivatives. In the absence of trapping agents, the xylylene relaxes to α,α'-dibromobenzocyclobutane:[2]
- C6H4(CHBr2)2 + 2 NaI → C6H4(=CHBr)2 + 2 NaBr + I2
- C6H4(=CHBr)2 → C6H4(CHBr)2
Cycloadditions of these o-xylylenes provides a pathway to acenes.[3]
The diene unit formed by the two exocyclic alkene units of the ortho isomer can serve as a ligand in coordination complexes. For example, reaction of α,α'-dibromo-o-xylene with iron carbonyls affords low yields of the xylylene complex Fe(CO)3[η4-C6H4(CH2)2]. This product is structurally analogous to Fe(CO)3[η4-1,3-butadiene].[4]
At high temperatures, benzocyclobutenes can undergo electrocyclic ring-opening to form o-xylylenes. This and other syntheses of o-xylylenes, and their subsequent dimerization by [4+4] cycloaddition to form cycloctyl structures, were used repeatedly in the synthesis of superphane.[5]
Electronic structure
Despite the observed chemistry of para-xylylene (i.e. its rapid polymerization to poly-p-xylylene), which suggests the compound exists as a diradical, physical evidence unanimously concludes that the lowest electronic state of p-xylylene is a closed shell singlet. Additionally, several computational methods confirm this assignment.[6] Conversely, meta-xylylene is a non-Kekulé molecule that has a triplet ground-state.[7]
References
- ^ H. E. Winberg, F. S. Fawcett "[2.2]Paracyclophane" Organic Syntheses, Coll. Vol. 5, p.883 (1973); Vol. 42, p.83 (1962) Link.
- ^ Cava, M. P.; Deana, A. A.; Muth, K. (1959). "Condensed Cyclobutane Aromatic Compounds. VIII. The Mechanism of Formation of 1,2-Dibromobenzocyclobutene; A New Diels-Alder Synthesis". Journal of the American Chemical Society. 81 (24): 6458–6460. doi:10.1021/ja01533a032.
- ^ Paddon-Row, Michael N.; Patney, Harish K. (1986). "An Efficient Synthetic Strategy for Naphthalene Annellation of Norbornenylogous Systems". Synthesis. 1986 (4): 328–330. doi:10.1055/s-1986-31603.
- ^ R. C. Kerber, E. C. Ribakove "Formation of iron carbonyl complexes of reactive polyenes from dihalides involving the free polyene" Organometallics, 1991, volume 10, pp 2848–2853.doi:10.1021/om00054a059
- ^ Sekine, Y.; Brown, M.; Boekelheide, V. (1979). "[2.2.2.2.2.2](1,2,3,4,5,6)Cyclophane: superphane". Journal of the American Chemical Society. 101 (11): 3126–3127. doi:10.1021/ja00505a053.
- ^ Montgomery, L. K., Huffman, J. C., Jurczak, E. A. & Grendze, M. P. The molecular structures of Thiele’s and Chichibabin’s hydrocarbons. J. Am. Chem. Soc. 108, 6004–6011 (1986) DOI:10.1021/ja00279a056
- ^ Steglich, Mathias; Custodis, Victoria B. F.; Trevitt, Adam J.; daSilva, Gabriel; Bodi, Andras; Hemberger, Patrick (2017). "Photoelectron Spectrum and Energetics of the meta-Xylylene Diradical". J. Am. Chem. Soc. 139 (41): 14348–14351. doi:10.1021/jacs.7b06714.