 | |
 | |
 | |
| Names | |
|---|---|
| Other names Disodium dioxide Flocool Solozone Disodium peroxide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.828 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1504 |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | Na2O2 |
| Molar mass | 77.98 g/mol |
| Appearance | yellow to white powder |
| Density | 2.805 g/cm3 |
| Melting point | 460 °C (860 °F; 733 K) (decomposes) |
| Boiling point | 657 °C (1,215 °F; 930 K) (decomposes) |
| reacts violently | |
| Solubility | soluble in acid insoluble in base reacts with ethanol |
| −28.10·10−6 cm3/mol | |
| Structure | |
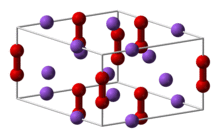
Crystal structure | hexagonal |
| Thermochemistry | |
Heat capacity (C) | 89.37 J/(mol·K) |
Std molar entropy (S | 95 J/(mol·K)[1] |
Std enthalpy of formation (ΔfH⦵298) | −515 kJ·mol−1[1] |
Gibbs free energy (ΔfG˚) | −446.9 kJ/mol |
| Hazards | |
| Safety data sheet | External MSDS |
EU classification (DSD) (outdated) | |
| R-phrases (outdated) | R8, R35 |
| S-phrases (outdated) | (S1/2), S8, S27, S39, S45 |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other cations | Lithium peroxide Potassium peroxide Rubidium peroxide Caesium peroxide |
| Sodium oxide Sodium superoxide | |
Related compounds | Sodium hydroxide Hydrogen peroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sodium peroxide is the inorganic compound with the formula Na2O2. This yellowish solid is the product of sodium ignited in excess oxygen.[3] It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and Na2O2·8H2O.[4] The octahydrate, which is simple to prepare, is white, in contrast to the anhydrous material.[5]
Properties
Sodium peroxide crystallizes with hexagonal symmetry.[6] Upon heating, the hexagonal form undergoes a transition into a phase of unknown symmetry at 512 °C.[7] With further heating above the 657 °C boiling point, the compound decomposes to Na2O, releasing O2.[8]
- 2 Na2O2 → 2 Na2O + O2
Preparation
The octahydrate is produced by treating sodium hydroxide with hydrogen peroxide.[5]
Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130–200 °C, a process that generates sodium oxide, which in a separate stage absorbs oxygen:[7][9]
- 4 Na + O2 → 2 Na2O
- 2 Na2O + O2 → 2 Na2O2
It may also be produced by passing ozone gas over solid sodium iodide inside a platinum or palladium tube. The ozone oxidizes the sodium to form sodium peroxide. The iodine can be sublimed by mild heating. The platinum or palladium catalyzes the reaction and is not attacked by the sodium peroxide.
Uses
Sodium peroxide hydrolyzes to give sodium hydroxide and hydrogen peroxide according to the reaction[9]
- Na2O2 + 2 H2O → 2 NaOH + H2O2
Sodium peroxide was used to bleach wood pulp for the production of paper and textiles. Presently it is mainly used for specialized laboratory operations, e.g., the extraction of minerals from various ores. Sodium peroxide may go by the commercial names of Solozone[7] and Flocool.[8] In chemistry preparations, sodium peroxide is used as an oxidizing agent. It is also used as an oxygen source by reacting it with carbon dioxide to produce oxygen and sodium carbonate:
- 2 Na2O2 + 2 CO2 → 2 Na2CO3 + O2
It is thus particularly useful in scuba gear, submarines, etc. Lithium peroxide has similar uses.
References
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 978-0-618-94690-7.
- ^ http://www.nmsu.edu/safety/programs/chem_safety/NFPA-ratingS-Z.htm
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 98. ISBN 978-0-08-022057-4.
- ^ Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort "Peroxo Compounds, Inorganic" Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_177.pub2.
- ^ a b R. A. Penneman (1950). "Potassium Sodium Peroxide 8-Hydrate". Inorg. Synth. 3: 1–4. doi:10.1002/9780470132340.ch1.
- ^ Tallman, R. L.; Margrave, J. L.; Bailey, S. W. (1957). "The Crystal Structure Of Sodium Peroxide". J. Am. Chem. Soc. 79 (11): 2979–80. doi:10.1021/ja01568a087.
- ^ a b c Macintyre, J. E., ed. Dictionary of Inorganic Compounds, Chapman & Hall: 1992.
- ^ a b Lewis, R. J. Sax's Dangerous Properties of Industrial Materials, 10th ed., John Wiley & Sons, Inc.: 2000.
- ^ a b E. Dönges "Lithium and Sodium Peroxides" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 979.
