| | |||
| Names | |||
|---|---|---|---|
| IUPAC name Sodium oxide | |||
| Other names Disodium oxide | |||
| Identifiers | |||
3D model (JSmol) | |||
| ECHA InfoCard | 100.013.827 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
| UN number | 1825 | ||
CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | Na2O | ||
| Molar mass | 61.979 g·mol−1 | ||
| Appearance | white solid | ||
| Density | 2.27 g/cm3 | ||
| Melting point | 1,132 °C (2,070 °F; 1,405 K) | ||
| Boiling point | 1,950 °C (3,540 °F; 2,220 K) sublimates | ||
Sublimation conditions | sublimates at 1275 °C | ||
| reacts violently to form NaOH | |||
| Solubility | reacts with ethanol | ||
| −19.8·10−6 cm3/mol | |||
| Structure | |||
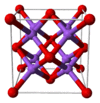
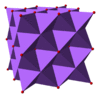
Crystal structure | Antifluorite (face centered cubic), cF12 | ||
| Fm3m, No. 225 | |||
| Tetrahedral (Na+); cubic (O2−) | |||
| Thermochemistry | |||
Heat capacity (C) | 72.95 J/(mol·K) | ||
Std molar entropy (S | 73 J/(mol·K)[1] | ||
Std enthalpy of formation (ΔfH⦵298) | −416 kJ/mol[1] | ||
Gibbs free energy (ΔfG˚) | −377.1 kJ/mol | ||
| Hazards | |||
| Main hazards | corrosive, reacts violently with water | ||
| Safety data sheet | ICSC 1653 | ||
| GHS pictograms |  [2] [2] | ||
GHS hazard statements | H314[2] | ||
GHS precautionary statements | P280[2] | ||
| NFPA 704 (fire diamond) | |||
| Flash point | non-flammable | ||
| Related compounds | |||
Other anions | Sodium sulfide Sodium selenide Sodium telluride | ||
Other cations | Lithium oxide Potassium oxide Rubidium oxide Caesium oxide | ||
| Sodium peroxide Sodium superoxide | |||
Related compounds | Sodium hydroxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Sodium oxide is a chemical compound with the formula Na2O. It is used in ceramics and glasses. The compound is the base anhydride of sodium hydroxide; when water is added to sodium oxide, NaOH is produced.
- Na2O + H2O → 2 NaOH
The alkali metal oxides M2O (M = Li, Na, K, Rb) crystallise in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in CaF2, with sodium ions tetrahedrally coordinated to 4 oxide ions and oxide cubically coordinated to 8 sodium ions.[3][4]
Preparation
Sodium oxide is produced by the reaction of sodium with sodium hydroxide, sodium peroxide, or sodium nitrite:[5]
- 2 NaOH + 2 Na → 2 Na2O + H2
- Na2O2 + 2 Na → 2 Na2O
- 2 NaNO2 + 6 Na → 4 Na2O + N2
Most of these reactions rely on the reduction of something by sodium, whether it is hydroxide, peroxide, or nitrite.
Burning sodium in air will produce Na2O and about 20% sodium peroxide Na2O2.
- 6 Na + 2 O2 → 2 Na2O + Na2O2
A more accessible way of producing it in the laboratory consists in decomposing the sodium salt of ascorbic acid at temperatures over 209 Celsius degrees.
Applications
Glassmaking
Sodium oxide is a significant component of most glass, although it is added in the form of "soda" (sodium carbonate). Typically, manufactured glass contains around 15% sodium oxide, 70% silica (silicon dioxide) and 9% lime (calcium oxide). The sodium carbonate "soda" serves as a flux to lower the temperature at which the silica mixture melts. Soda glass has a much lower melting temperature than pure silica, and has slightly higher elasticity. These changes arise because the silicon dioxide and soda have reacted to form sodium silicates of the general formula Na2[SiO2]x[SiO3].
- Na2CO3 → Na2O + CO2
- Na2O + SiO2 → Na2SiO3
References
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- ^ a b c Sigma-Aldrich Co., Sodium oxide. Retrieved on 2014-05-25.
- ^ Zintl, E.; Harder, A.; Dauth B. (1934). "Gitterstruktur der oxyde, sulfide, selenide und telluride des lithiums, natriums und kaliums". Z. Elektrochem. Angew. Phys. Chem. 40: 588–93. doi:10.1002/bbpc.19340400811.
- ^ Wells, A. F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.