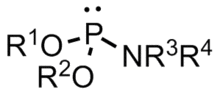A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids e.c., triethylammonium chloride or 1H-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety.
Applications
Nucleoside phosphoramidites
Phosphoramidites derived from protected nucleosides are referred to as nucleoside phosphoramidites and are widely used in chemical synthesis of DNA, RNA, and other nucleic acids and their analogs.
As ligands
Certain phosphoramidites are also used as monodentate chiral ligands in asymmetric synthesis.[1] A large group of such ligands is derived from the chiral diol BINOL and can be synthesised by reaction of BINOL with phosphorus trichloride to the chlorophosphite and then reaction with simple secondary amines.[2] This type of ligand was first used in 1996 in an asymmetric copper-catalysed addition of dialkylzincs to enones [3][4]
See also
- Phosphoramidate
References
- ^ Teichert, J.; Feringa, B. (2010). "Phosphoramidites: Privileged Ligands in Asymmetric Catalysis". Angewandte Chemie International Edition in English. 49 (14): 2486–2528. doi:10.1002/anie.200904948. PMID 20333685.
- ^ Hulst, R.; De Vries, N. K.; Feringa, B. L. (1994). "α-Phenylethylamine based chiral phospholidines; new agents for the determination of the enantiomeric excess of chiral alcohols, amines and thiols by means of 31P NMR". Tetrahedron: Asymmetry. 5 (4): 699–708. doi:10.1016/0957-4166(94)80032-4.
- ^ De Vries, A. H. M.; Meetsma, A.; Feringa, B. L. (1996). "Enantioselective Conjugate Addition of Dialkylzinc Reagents to Cyclic and Acyclic Enones Catalyzed by Chiral Copper Complexes of New Phosphorus Amidites" (PDF). Angewandte Chemie International Edition in English. 35 (20): 2374. doi:10.1002/anie.199623741.
- ^ Feringa, B. L.; Pineschi, M.; Arnold, L. A.; Imbos, R.; De Vries, A. H. M. (1997). "Highly Enantioselective Catalytic Conjugate Addition and Tandem Conjugate Addition–Aldol Reactions of Organozinc Reagents" (PDF). Angewandte Chemie International Edition in English. 36 (23): 2620. doi:10.1002/anie.199726201.