 | |
 | |
| Names | |
|---|---|
| IUPAC name (2R,3R,4S,5S,6R)-2-[(2S,3S,4S,5R)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-6-[[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxane-3,4,5-triol | |
| Other names rafinosa D-(+)-Raffinose D-Raffinose D-raffinose pentahydrate Gossypose Melitose Melitriose NSC 170228 NSC 2025 6G-α-D-galactosylsucrose; β-D-fructofuranosyl-O-α-D-glucopyranosyl-(1→6)-α-D-galactopyranoside hydrate(1:5) | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.407 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | C18H32O16 |
| Molar mass | 594.5 g/mol (pentahydrate) |
| Melting point | 118 °C |
| 203 g/L | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
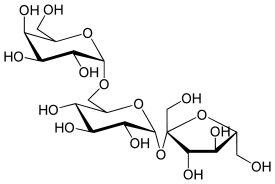
Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme not found in the human digestive tract. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. The enzyme does not cleave β-linked galactose, as in lactose.
Chemical properties
The raffinose family of oligosaccharides (RFOs) are alpha-galactosyl derivatives of sucrose, and the most common are the trisaccharide raffinose, the tetrasaccharide stachyose, and the pentasaccharide verbascose. RFOs are almost ubiquitous in the plant kingdom, being found in a large variety of seeds from many different families, and they rank second only to sucrose in abundance as soluble carbohydrates.
Raffinose may have a form of a white crystalline powder. It is odorless and has a sweet taste approximately 10% that of sucrose.[1]
Biochemical properties
Energy source
It is non-digestible in humans and other monogastric animals (pigs and poultry) who do not possess the α-GAL enzyme to break down RFOs. These oligosaccharides pass undigested through the stomach and small intestine. In the large intestine, they are fermented by bacteria that do possess the α-GAL enzyme and make short-chain fatty acids (SCFA)(acetic, propionic, butyric acids), as well as the flatulence commonly associated with eating beans and other vegetables. These SCFAs have been recently found to impart a number of health benefits. α-GAL is present in digestive aids such as the product Beano.
Disease relevance
Research has shown that the differential ability to utilize raffinose by strains of the bacteria Streptococcus pneumoniae, impacts their ability to cause disease.[2]
Uses
Procedures concerning cryopreservation have used raffinose to provide hypertonicity for cell desiccation prior to freezing.[3] Either raffinose or sucrose is used as a base substance for sucralose.
Raffinose is also used in:[1]
- skin moisturizers and smoothers
- prebiotics (it allegedly promotes growth of lactobacilli and bifidobacteria)[4]
- food or drinks additive
See also
- Raffinose—raffinose alpha-galactosyltransferase
Further reading
References
- ^ a b "D(+)-Raffinose pentahydrate | 17629-30-0". www.chemicalbook.com. Retrieved 2019-08-19.
- ^ Minhas, Vikrant; Harvey, Richard M.; McAllister, Lauren J.; Seemann, Torsten; Syme, Anna E.; Baines, Sarah L.; Paton, James C.; Trappetti, Claudia (2019-01-15). McDaniel, Larry S. (ed.). "Capacity To Utilize Raffinose Dictates Pneumococcal Disease Phenotype". mBio. 10 (1). doi:10.1128/mBio.02596-18. ISSN 2150-7511.
- ^ Storey B., Noiles, E., Thompson, K. (1998). "Comparison of Glycerol, Other Polyols, Trehalose, and Raffinose to Provide a Defined Cryoprotectant Medium for Mouse Sperm Cryopreservation". Cryobiology. 37 (1): 46–58. doi:10.1006/cryo.1998.2097. PMID 9698429.
- ^ Zartl B, Silberbauer K, Loeppert R, Viernstein H, Praznik W, Mueller M. Fermentation of non-digestible raffinose family oligosaccharides and galactomannans by probiotics. Food Funct. 2018 Mar 1;9(3):1638-1646. doi: 10.1039/c7fo01887h. Epub 2018 Feb 21. PMID: 29465