 | |
 | |
| Names | |
|---|---|
| IUPAC name Dimethyl but-2-ynedioate | |
| Other names DMAD Acetylenedicarboxylic acid dimethyl ester | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.999 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C6H6O4 | |
| Molar mass | 142.11 g/mol |
| Appearance | Colorless liquid |
| Density | 1.1564 g/cm3 |
| Melting point | -18°C |
| Boiling point | 195 to 198 °C (383 to 388 °F; 468 to 471 K) (96–98° at 8 mm Hg) |
| Insoluble | |
| Solubility in other solvents | Soluble in most organic solvents |
Refractive index (nD) | 1.447 |
| Structure | |
| 0 D | |
| Hazards | |
| Main hazards | Toxic gas |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements | H302, H314 |
GHS precautionary statements | P260, P264, P270, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P405, P501 |
| Flash point | 187 °C (369 °F; 460 K) |
| Related compounds | |
Related compounds | Methyl propiolate, Hexafluoro-2-butyne, Acetylene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
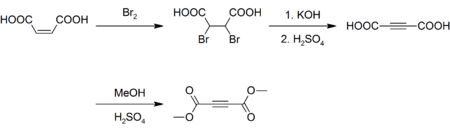
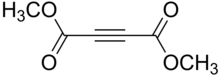
Dimethyl acetylenedicarboxylate (DMAD) is an organic compound with the formula CH3O2CC2CO2CH3. It is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed as a dienophile in cycloaddition reactions, such as the Diels-Alder reaction. It is also a potent Michael acceptor.[1][2] This compound exists as a colorless liquid at room temperature. This compound was used in the preparation of nedocromil.
Preparation
Although inexpensively available, DMAD is prepared today as it was originally. Maleic acid is brominated and the resulting dibromosuccinic acid is dehydrohalogenated with potassium hydroxide yielding acetylenedicarboxylic acid.[3][4] The acid is then esterified with methanol and sulfuric acid as a catalyst:[5]
Safety
DMAD is a lachrymator and a vesicant.
References
- ^ Stelmach, J. E.; Winkler, J. D. "Dimethyl Acetylenedicarboxylate"in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.
- ^ Sahoo, Manoj (2007). "Dimethyl Acetylene Dicarboxylate". Synlett. 2007 (13): 2142–2143. doi:10.1055/s-2007-984894.
- ^ Bandrowski, E. (1877). "Ueber Acetylendicarbonsäure". Berichte der Deutschen Chemischen Gesellschaft. 10: 838–842. doi:10.1002/cber.187701001231.
- ^ Abbott, T. W.; Arnold, R. T.; Thompson, R. B. "Acetylenedicarboxylic acid". Organic Syntheses.; Collective Volume, 2, p. 10
- ^ Huntress, E. H.; Lesslie, T. E.; Bornstein, J. "Dimethyl Acetylenedicarboxylate". Organic Syntheses.; Collective Volume, 4, p. 329