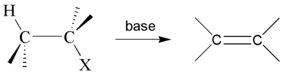
Dehydrohalogenation is an elimination reaction that eliminates (removes) a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.
Dehydrohalogenation from alkyl halides
Traditionally, alkyl halides are substrates for dehydrohalogenations. The alkyl halide must be able to form an alkene, thus methyl and benzy halides are not suitable substrates. Aryl halides are also unsuitable. Upon treatment with strong base, chlorobenzene dehydrohalogenates to give phenol via a benzyne intermediate.
Base-promoted reactions
When treated with a strong base many alkyl chlorides convert to corresponding alkene.[1] It is also called a β-elimination reaction and is a type of elimination reaction. Some examples are shown below:
Here ethyl chloride reacts with potassium hydroxide, typically in a solvent such as ethanol, giving ethylene. Likewise, 1-chloropropane and 2-chloropropane give propene.
Zaitsev's rule helps to predict regioselectivity for this reaction type.
In general, the reaction of a haloalkane with potassium hydroxide can compete with an SN2 nucleophilic substitution reaction by OH− a strong, unhindered nucleophile. Alcohols are however generally minor products. Dehydrohalogenations often employ strong bases such as potassium tert-butoxide (K+ [CH3]3CO−).
Thermal cracking
On an industrial scale, base-promoted dehydrohalogenations as described above are disfavored. The disposal of the alkali halide salt is problematic. Instead thermally-induced dehydrohalogenations are preferred. One example is provided by the production of vinyl chloride by heating 1,2-dichloroethane:[2]
- CH2Cl-CH2Cl → CH2=CHCl + HCl
The resulting HCl can be reused in oxychlorination reaction.
Thermally induced dehydrofluorinations are employed in the production of fluoroolefins and hydrofluoroolefins. One example is the preparation of 1,2,3,3,3-pentafluoropropene from 1,1,2,3,3,3-hexafluoropropane:
- CF2HCH(F)CF3 → CHF=C(F)CF3 + HF
Other dehydrohalogenations
Epoxides
Chlorohydrins, compounds with the connectivity R(HO)CH-CH(Cl)R', undergo dehydrochlorination to give epoxides. This reaction is employed industrially to produce millions of tons of propylene oxide annually from propylene chlorohydrin:[3]
- CH3CH(OH)CH2Cl + KOH → CH3CH(O)CH2 + H2O + KCl
Isocyanides
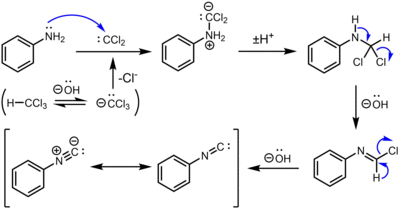
The carbylamine reaction for the synthesis of isocyanides from the action of chloroform on a primary amine involves three dehydrohalogenations. The first dehydrohalogenation is the formation of dichlorocarbene:
- KOH + CHCl3 → KCl + H2O + CCl2
Two successive base-mediated dehydrochlorination steps result in formation of the isocyanide.[4]
External links
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ^ M. Rossberg et al. "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ^ Nijhuis, T. Alexander; Makkee, Michiel; Moulijn, Jacob A.; Weckhuysen, Bert M. "The Production of Propene Oxide: Catalytic Processes and Recent Developments" Industrial & Engineering Chemistry Research 2006, volume 45, 3447-3459. doi:10.1021/ie0513090
- ^ Gokel, G.W.; Widera, R.P.; Weber, W.P. (1988). "Phase-transfer Hofmann carbylamine reaction: tert-butyl isocyanide". 55: 232. doi:10.15227/orgsyn.055.0096.