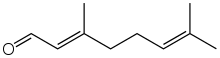
 Geranial | |
 | |
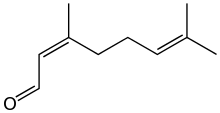 Neral | |
 | |
| Names | |
|---|---|
| IUPAC name 3,7-dimethylocta-2,6-dienal | |
| Other names citral geranialdehyde | |
| Identifiers | |
3D model (JSmol) | |
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.023.994 |
| EC Number |
|
IUPHAR/BPS | |
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2810 |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | C10H16O |
| Molar mass | 152.24 g/mol |
| Appearance | Pale yellow liquid |
| Odor | Lemon like |
| Density | 0.893 g/cm3 |
| Boiling point | 229 °C (444 °F; 502 K) |
| Vapor pressure | 0.22 mmHg (20 °C) |
| −98.9×10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements | H315, H317 |
GHS precautionary statements | P261, P264, P272, P280, P302+352, P321, P332+313, P333+313, P362, P363, P501 |
| NFPA 704 (fire diamond) | |
| Flash point | 91 °C (196 °F; 364 K) |
| Related compounds | |
Related alkenals |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Citral, or 3,7-dimethyl-2,6-octadienal or lemonal, is either a pair, or a mixture of terpenoids with the molecular formula C10H16O. The two compounds are geometric isomers. The E-isomer is known as geranial or citral A. The Z-isomer is known as neral or citral B.
Occurrence
Citral is present in the oils of several plants, including lemon myrtle (90–98%), Litsea citrata (90%), Litsea cubeba (70–85%), lemongrass (65–85%), lemon tea-tree (70–80%), Ocimum gratissimum (66.5%), Lindera citriodora (about 65%), Calypranthes parriculata (about 62%), petitgrain (36%), lemon verbena (30–35%), lemon ironbark (26%), lemon balm (11%), lime (6–9%), lemon (2–5%), and orange.[2][3][4]
Uses
Citral has a strong lemon (citrus) odor and is used as an aroma compound in perfumery. (Nerol, another perfumery compound, has a less intense but sweeter lemon odor.) In addition, Citral is used as a flavor and for fortifying lemon oil. It also has strong antimicrobial qualities,[5] and pheromonal effects in acari and insects.[6][7]
Citral is used in the synthesis of vitamin A, lycopene, ionone, and methylionone, to mask the smell of smoke.
Health and safety information
Two studies showed 1–1.7% of people to be allergic to citral, with allergies frequently reported. Citral on its own is strongly sensitizing to allergies; the International Fragrance Association recommends that citral only be used in association with substances that prevent a sensitizing effect. Citral has been extensively tested, with no known genotoxicity or carcinogenic effect.[8]
See also
References
- ^ Citral, The Merck Index, 12th Edition.
- ^ Fenaroli, G., Furia, T.E., Bellanca, N., Handbook of Flavor Ingredients, ISBN 0-87819-532-7
- ^ Lawless, J., The Illustrated Encyclopedia of Essential Oils, ISBN 1-85230-661-0
- ^ The Aromatic Plant Project
- ^ Onawunmi, G.O. (1989). "Evaluation of the antimicrobial activity of citral". Lett. Appl. Microbiol. 9 (3): 105–108. doi:10.1111/j.1472-765X.1989.tb00301.x.
- ^ Kuwahara, Yasumasa; Suzuki, Hiroshi; Matsumoto, Katsuhiko; Wada, Yoshitake (1983). "Pheromone study on acarid mites. XI. Function of mite body as geometrical isomerization and reduction of citral (the alarm pheromone)". Applied Entomology and Zoology. 18 (1): 30–39. doi:10.1303/aez.18.30.
- ^ Robacker, D.C.; Hendry, L.B. (1977). "Neral and geranial: components of the sex pheromone of the parasitic wasp, Itoplectis conquisitor". J. Chem. Ecol. 3 (5): 563–577. doi:10.1007/BF00989077.
- ^ Survey and health assessment of chemical substances in massage oils Archived 28 June 2007 at the Wayback Machine
