 | |
| Names | |
|---|---|
| IUPAC name Triphenylstibine | |
| Other names Triphenylantimony | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.009.125 |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C18H15Sb |
| Molar mass | 353.07 g/mol |
| Appearance | Colourless solid |
| Density | 1.53 g/cm3 |
| Melting point | 52 to 54 °C (126 to 129 °F; 325 to 327 K) |
| Boiling point | 377 °C (711 °F; 650 K) |
| insoluble | |
| Structure | |
| trigonal pyramidal | |
| Hazards | |
| Main hazards | mildly toxic |
| R-phrases (outdated) | 20/22-51/53 |
| S-phrases (outdated) | 61 |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related compounds | Triphenylphosphine Triphenylarsine Stibine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
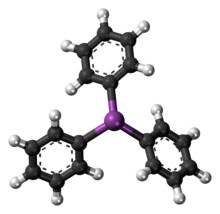
Triphenylstibine is the chemical compound with the formula Sb(C6H5)3. Abbreviated SbPh3, this colourless solid is often considered the prototypical organoantimony compound. It is used as a ligand in coordination chemistry[1] and as a reagent in organic synthesis.
Like the related molecules triphenylphosphine and triphenylarsine, SbPh3 is pyramidal with a propeller-like arrangement of the phenyl groups. The Sb-C distances average 2.14-2.17 Å and the C-Sb-C angle are 95°.[2]
SbPh3 was first reported in 1886, being prepared from antimony trichloride by the reaction:[3]
- 6 Na + 3 C6H5Cl + SbCl3 → (C6H5)3Sb + 6 NaCl
An alternative method treats phenylmagnesium bromide with SbCl3.[4]
References
- ^ C. A. McAuliffe, ed. (1973). Transition Metal Complexes of Phosphorus, Arsenic, and Antimony Ligands. J. Wiley. ISBN 0-470-58117-4.
- ^ Adams, E. A.; Kolis, J. W.; Pennington, W. T. "Structure of triphenylstibine" Acta Crystallographica 1990, volume C46, pp. 917-919. doi:10.1107/S0108270189012862
- ^ Michaelis, A.; Reese, A. "Ueber die Verbindungen der Elemente der Stickstoffgruppe mit den Radicalen der aromatischen Reihe. Achte Abhandlung Ueber aromatische Antimonverbindungen" Liebigs Annallen der Chemie volume 233, pages 39-60 (1886). doi:10.1002/jlac.18862330104.
- ^ Hiers, G. S. (1927). "Triphenylstibine". Organic Syntheses. 7: 80. doi:10.15227/orgsyn.007.0080.
