 | |||
| | |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.016 | ||
PubChem CID | |||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
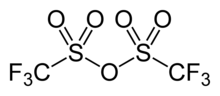
Chemical formula | C2F6O5S2 | ||
| Molar mass | 282.13 g·mol−1 | ||
| Appearance | colourless liquid | ||
| Density | 1.6770 g/mL | ||
| Boiling point | 82[1] °C (180 °F; 355 K) | ||
| Hazards | |||
| Safety data sheet | Fisher MSDS | ||
| R-phrases (outdated) | R14 R34 | ||
| S-phrases (outdated) | S25 S26 S36/37/39 S45 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Trifluoromethanesulfonic anhydride, also known as triflic anhydride, is the chemical compound with the formula (CF3SO2)2O. It is the acid anhydride derived from triflic acid. This compound is a strong electrophile, useful for introducing the triflyl group, CF3SO2. Abbreviated Tf2O, triflic anhydride is the acid anhydride of the strong acid triflic acid, CF3SO2OH.[2][3]
Preparation and uses
Triflic anhydride is prepared by dehydration of triflic acid using P4O10.[2]
Triflic anhydride is useful for converting ketones into enol triflates.[4]
In a representative application, is used to convert an imine into a NTf group.[5] It will convert phenols into a triflic ester, which enables cleavage of the C-O bond.[6][7]
Assay
The typical impurity in triflic anhydride is triflic acid, which is also a colorless liquid. Samples of triflic anhydride can be assayed by 19F NMR spectroscopy: −72.6 ppm[8] vs. −77.3 for TfOH (std CFCl3).
Safety
It is an aggressive electrophile and readily hydrolyzes to the strong acid triflic acid. It is very harmful to skin and eyes.[9]
See also
- Methanesulfonic anhydride
References
- ^ Bloodworth, A.J.; Curtis, Richard J.; Spencer, Michael D.; Tallant, Neil A. (March 1993). "Oxymetallation. Part 24. Preparation of cyclic peroxides by cycloperoxymercuriation of unsaturated hydroperoxides". Tetrahedron. 49 (13): 2729–2750. doi:10.1016/S0040-4020(01)86350-X.
- ^ a b Martínez, A. G.; Subramanian, L. R.; Hanack, M. (2016). "Trifluoromethanesulfonic Anhydride". Encyclopedia of Reagents for Organic Synthesis: 1–17. doi:10.1002/047084289X.rt247.pub3. ISBN 9780470842898.
- ^ Baraznenok, Ivan L.; Nenajdenko, Valentine G.; Balenkova, Elizabeth S. (May 2000). "Chemical Transformations Induced by Triflic Anhydride". Tetrahedron. 56 (20): 3077–3119. doi:10.1016/S0040-4020(00)00093-4.
- ^ Cacchi, Sandro; Morera, Enrico; Ortar, Giorgio (2011). "Discussion Addendum for: Palladium-Catalyzed Reduction of Vinyl Trifluoromethanesulfonates to Alkenes: Cholesta-3,5-diene". Organic Syntheses. 88: 260. doi:10.15227/orgsyn.088.0260.
- ^ Baker, T. J.; Tomioka, M.; Goodman, M. (2002). "Preparation and Use of N,N'-Di-BOC-N''-Triflylguanidine". Organic Syntheses. 78: 91. doi:10.15227/orgsyn.078.0091.
- ^ McWilliams, J. C.; Fleitz, F. J.; Zheng, N.; Armstrong, III, J. D. "Preparation of n-Butyl 4-Chlorophenyl Sulfide". Organic Syntheses. 79: 43. doi:10.15227/orgsyn.079.0043.
- ^ Cai, D.; Payack, J. F.; Bender, D. R.; Hughes, D. L.; Verhoeven, T. R.; Reider, P. J. (1999). "(R)-(+)- and (S)-(−)-2,2'-Bis(Diphenylphosphino)-1,1'-Binaphthyl (BINAP)". Organic Syntheses. 76: 6. doi:10.15227/orgsyn.076.0006.
- ^ Dell'Amico, Daniela Belli; Boschi, Daniele; Calderazzo, Fausto; Labella, Luca; Marchetti, Fabio (28 February 2002). "Synthesis, and crystal and molecular structures of the triflato and trifluoroacetato complexes of zinc, Zn(O3SCF3)2(DME)2 and [Zn(O2CCF3)2(DME)]n" (PDF). Inorganica Chimica Acta. 330 (1): 149–154. doi:10.1016/S0020-1693(01)00739-3.
- ^ "MSDS - 176176". www.sigmaaldrich.com. Retrieved 2020-08-09.