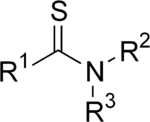
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure R–CS–NR′R″, where R, R′, and R″ are organic groups. They are analogous to amides but they exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier.[1] One of the best-known thioamides is thioacetamide, which is used as a source of the sulfide ion and is a building block in heterocyclic chemistry.
Thioamides or anti-thyroid drugs are also a class of drugs that are used to control thyrotoxicosis.
Preparation and structure
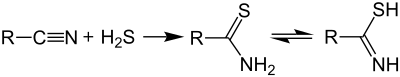
Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide and, in more specialized applications, Lawesson's reagent.[2][3] An alternative route entails the reaction of nitriles with hydrogen sulfide:
The Willgerodt-Kindler reaction also affords benzylthioamides.[4]
The C2NH2S core of thioamides is planar. Using thioacetamide as representative: the C-S, C-N, and C-C distances are 1.68, 1.31, and 1.50 Å, respectively. The short C-S and C-N distances indicate multiple bonding.[5]
Thioamides in biochemistry and medicine
Thioamides are also a class of drugs that are used to control thyrotoxicosis.
Thioamides have been incorporated into peptides as isosteres for the amide bond.[6] Peptide modifications are analogues of the native peptide, which can reveal the structure-activity relationship (SAR). Analogues of peptides can also be used as drugs with an improved oral bioavailability. Thioamides inhibit the enzyme thyroid peroxidase in the thyroid, reducing the synthesis of triiodothyronine (T3) and thyroxine (T4), thereby blocking uptake of iodotyrosines from the colloid. They also block iodine release from peripheral hormone. Maximum effects occur only after a month, since hormone depletion is caused by reduced synthesis, which is a slow process.
Because thioamides can penetrate the placental barrier, caution is advised when used during pregnancy. Ten percent of patients report skin eruptions (such as macules and papules), urticaria, dermatitis, fever, and arthralgia; 0.03% of all patients develop agranulocytosis, aplastic anemia also a rare adverse effect.
Members of the thioamide group include methimazole, carbimazole (converted in vivo to methimazole), and propylthiouracil.
References
- ^ Wiberg, Kenneth B.; Rablen, Paul R. (1995). "Why Does Thioformamide Have a Larger Rotational Barrier Than Formamide?". J. Am. Chem. Soc. 117 (8): 2201–2209. doi:10.1021/ja00113a009.
- ^ Gompper, R.; Elser, W. (1973). "2-Methylmercapto-N-Methyl-Δ2-Pyrroline". Organic Syntheses.; Collective Volume, 5, p. 780
- ^ Schwarz, George (1955). "2,4-Dimethylthiazole". Organic Syntheses.; Collective Volume, 3, p. 332
- ^ Rolfs, Andreas; Liebscher, Jürgen (1998). "3-Morpholino-2-Phenylthioacrylic Acid Morpholide and 5-(4-Bromobenzoyl-2-(4-Morpholino)-3-Phenylthiophene". Organic Syntheses.; Collective Volume, 9, p. 99
- ^ Trevor W. Hambley, David E. Hibbs, Peter Turner, Siân. T. Howard, Michael B. Hursthouse (2002). "Insights into Bonding and Hydrogen Bond Directionality in Thioacetamide from the Experimental Charge Distribution". J. Chem. Soc., Perkin Trans. (2): 235–239. doi:10.1039/B109353C.
- ^ Artis, Dean R.; Lipton, Mark A. (1998). "Conformations of Thioamide-Containing Dipeptides: A Computational Study". J. Am. Chem. Soc. 120 (47): 12200–12206. doi:10.1021/ja982398t.