Thiamine monophosphate 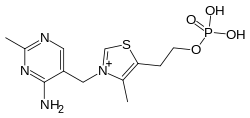 |
| Identifiers |
| | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.762  |
| MeSH | Thiamine+Monophosphate |
| | |
| | |
InChI=1S/C12H17N4O4PS.ClH/c1-8-11(3-4-20-21(17,18)19)22-7-16(8)6-10-5-14-9(2)15-12(10)13;/h5,7H,3-4,6H2,1-2H3,(H3-,13,14,15,17,18,19);1H  Key: GUGWNSHJDUEHNJ-UHFFFAOYSA-N  InChI=1/C12H17N4O4PS.ClH/c1-8-11(3-4-20-21(17,18)19)22-7-16(8)6-10-5-14-9(2)15-12(10)13;/h5,7H,3-4,6H2,1-2H3,(H3-,13,14,15,17,18,19);1H Key: GUGWNSHJDUEHNJ-UHFFFAOYAK
|
SMILES Cc1c(sc[n+]1Cc2cnc(nc2N)C)CCOP(=O)(O)O.[Cl-]
|
| Properties |
Chemical formula | C12H18N4O4PS+ |
| Molar mass | 345.336 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
| | |
Thiamine monophosphate is a thiamine derivative. It occurs naturally in milk.[1]
References
- ^ Schmidt, Anatol; Pratsch, Herbert; Schreiner, Maximilian G.; Mayer, Helmut K. (2017). "Determination of the native forms of vitamin B1 in bovine milk using a fast and simplified UHPLC method". Food Chemistry. 229: 452–457. doi:10.1016/j.foodchem.2017.02.092. PMID 28372200.
This article is copied from an
article on Wikipedia® - the free encyclopedia created and edited by its online user community. The text was not checked or edited by anyone on our staff. Although the vast majority of Wikipedia® encyclopedia articles provide accurate and timely information, please do not assume the accuracy of any particular article. This article is distributed under the terms of
GNU Free Documentation License.
All content on this website, including dictionary, thesaurus, literature, geography, and other reference data is for informational purposes only. This information should not be considered complete, up to date, and is not intended to be used in place of a visit, consultation, or advice of a legal, medical, or any other professional.
