 | |
| Names | |
|---|---|
| Other names thallium azide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | TlN3 |
| Molar mass | 246.4035 |
| Appearance | yellow-brown |
| insoluble | |
| Structure | |
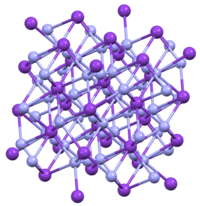
Crystal structure | Tetragonal, tI16 [1] |
| I4/mcm, No. 140 | |
| Hazards | |
| Main hazards | very toxic |
EU classification (DSD) (outdated) | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Thallium azide, TlN3, is a yellow-brown crystalline solid poorly soluble in water. Although it is not nearly as sensitive to shock or friction as lead azide, it can easily be detonated by a flame or spark. It can be stored safely dry in a closed non-metallic container.
Preparation and structure
Thallium azide can be prepared treating an aqueous solution of thallium(I) sulfate with sodium azide. Thallium azide will precipitate; the yield can be maximized by cooling.
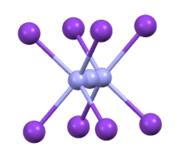
TlN3, KN3, RbN3, and CsN3 adopt the same structures. The azide is bound to eight cations in an eclipsed orientation. The cations are bound to eight terminal N centers.[2]
Safety
All thallium compounds are poisonous and should be handled with care; avoid breathing any dust or fumes.
References
- ^ Mauer F.A.; Hubbard C.R.; Hahn T.A. (1973). "Thermal expansion and low temperature phase transition of thallous azide". J. Chem. Phys. 59 (7): 3770–3776. doi:10.1063/1.1680549.
- ^ Ulrich Müller "Verfeinerung der Kristallstrukturen von KN3, RbN3, CsN3 und TIN3" Zeitschrift für anorganische und allgemeine Chemie 1972, Volume 392, 159–166. doi:10.1002/zaac.19723920207
| HN3 | He | ||||||||||||||||||
| LiN3 | Be(N3)2 | B(N3)3 | CH3N3, C(N3)4 | N(N3)3,H2N—N3 | O | FN3 | Ne | ||||||||||||
| NaN3 | Mg(N3)2 | Al(N3)3 | Si(N3)4 | P | SO2(N3)2 | ClN3 | Ar | ||||||||||||
| KN3 | Ca(N3)2 | Sc(N3)3 | Ti(N3)4 | VO(N3)3 | Cr(N3)3, CrO2(N3)2 | Mn(N3)2 | Fe(N3)2, Fe(N3)3 | Co(N3)2, Co(N3)3 | Ni(N3)2 | CuN3, Cu(N3)2 | Zn(N3)2 | Ga(N3)3 | Ge | As | Se(N3)4 | BrN3 | Kr | ||
| RbN3 | Sr(N3)2 | Y | Zr(N3)4 | Nb | Mo | Tc | Ru(N3)63− | Rh(N3)63− | Pd(N3)2 | AgN3 | Cd(N3)2 | In | Sn | Sb | Te | IN3 | Xe(N3)2 | ||
| CsN3 | Ba(N3)2 | Hf | Ta | W | Re | Os | Ir(N3)63− | Pt(N3)62− | Au(N3)4− | Hg2(N3)2, Hg(N3)2 | TlN3 | Pb(N3)2 | Bi(N3)3 | Po | At | Rn | |||
| Fr | Ra(N3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(N3)3, Ce(N3)4 | Pr | Nd | Pm | Sm | Eu | Gd(N3)3 | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||||
| Ac | Th | Pa | UO2(N3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||