| | |||
| Names | |||
|---|---|---|---|
| IUPAC name Tetrahydroxyborate | |||
| Systematic IUPAC name | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
Gmelin Reference | 1966 | ||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
SMILES
| |||
| Properties | |||
| H4BO4− | |||
| Molar mass | 78.840 g mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
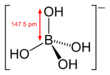
Tetrahydroxyborate (systematically named tetrahydroxyboranuide and tetrahydroxidoborate(1−)) is an inorganic anion with the chemical formula [B(OH)
4]−
(also written as B(OH)−
4 or BH
4O−
4). It contributes no colour to tetrahydroxyborate salts. It is found in the mineral hexahydroborite, Ca(B(OH)4)2 • 2H2O (originally formulated CaB2O4 • 6H2O).[2] It is classified as a weak base.
Chemical properties
Basicity
Tetrahydroxyborate can assimilate a proton into the anion by recombination:
-
(1)
Because of this capture of a proton (H+
), tetrahydroxyborate has Arrhenius-basic character. Its protonation product is boric acid. In aqueous solution, most tetrahydroxyborate ions are undissociated.
-
(2)
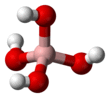
Structure
It is a boron oxoanion with a tetrahedral geometry.[3] It is isoelectronic with the hypothetical compound orthocarbonic acid.
Chemical reactions
Tetrahydroxyborate undergoes the typical chemical reactions of a hydroxyborate. Upon treatment with a standard acid, it converts to boric acid and a metal salt. Oxidation of tetrahydroxyborate gives perborate. When heated to a high temperature, tetrahydroxyborate salts decompose to produce metaborate salts and water, or to produce boric acid and a metal hydroxide:
- n [B(OH)4]− → (BO−
2)n + 2n H
2O - [B(OH)4]− → B(OH)3 + HO−
Production
Tetrahydroxyborate is produced by boric acid alkalinisation. In this process boric acid and hydroxide anions react to produce tetrahydroxyborate according to the following reaction:
- B(OH)3 + HO− → [B(OH)4]−
This process also involves aquatrihydroxyboron as an intermediate, and occurs in two steps. No catalyst is needed for alkalinisation (step 2).
Catalytic amounts of water are used for the process. By altering the process conditions, other borate anions may also be produced on the same production site.
Uses
Tetrahydroxyborate can be used as a cross-link in polymers.
Occurrence
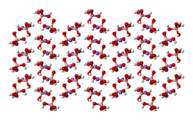
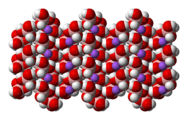
The tetrahydroxyborate anion is found in Na[B(OH)4],[4] Na2[B(OH)4]Cl and CuII[B(OH)4]Cl.
ball-and-stick model of the crystal
structure of sodium tetrahydroxyboratespace-filling model of the crystal
structure of sodium tetrahydroxyborate
See also
References
- ^ a b "Tetrahydroxoborate(1-) (CHEBI:41132)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ^ Glossary of Geology,5th edition, 2005, ISBN 978-0922152766 ed. by Julia A. Jackson, James P. Mehl, Klaus K. E. Neuendorf, American Geological Institute
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 203–205. ISBN 978-0-08-037941-8.
- ^ L. J. Csetenyi; F. P. Glasser; R. A. Howie (June 1993). "Structure of sodium tetrahydroxyborate". Acta Crystallogr. C. 49 (6): 1039–1041. doi:10.1107/S0108270193000058.