| | |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) | |||
| ECHA InfoCard | 100.029.115 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
SMILES
| |||
| Properties | |||
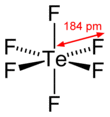
Chemical formula | TeF6 | ||
| Molar mass | 241.590 g/mol | ||
| Appearance | colorless gas | ||
| Odor | repulsive odor | ||
| Density | 0.0106 g/cm3 (-10 °C) 4.006 g/cm3 (-191 °C) | ||
| Melting point | −38.9 °C (−38.0 °F; 234.2 K)[2] | ||
| Boiling point | −37.6 °C (−35.7 °F; 235.6 K)[2] | ||
| decomposes | |||
| Vapor pressure | >1 atm (20°C)[1] | ||
| −66.0·10−6 cm3/mol | |||
Refractive index (nD) | 1.0009 | ||
| Structure | |||
Crystal structure | Orthorhombic, oP28 | ||
| Pnma, No. 62 | |||
| octahedral (Oh) | |||
Dipole moment | 0 | ||
| Thermochemistry | |||
Heat capacity (C) | 117.6 J/(mol K) | ||
Std enthalpy of formation (ΔfH⦵298) | -1318 kJ/mol | ||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published) | 5 ppm (rat, 4 hr) 5 ppm (mouse, 1 hr) 5 ppm (rabbit, 4 hr) 5 ppm (guinea pig, 4 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 0.02 ppm (0.2 mg/m3)[1] | ||
REL (Recommended) | TWA 0.02 ppm (0.2 mg/m3)[1] | ||
IDLH (Immediate danger) | 1 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Tellurium hexafluoride is a chemical compound of tellurium and fluorine with the chemical formula TeF6. It is a colorless, highly toxic gas with an extremely unpleasant smell.
Preparation
Tellurium hexafluoride is most commonly prepared by passing fluorine gas over tellurium at 150 °C. Below this temperature a mixture of lower fluorides form, including tellurium tetrafluoride. It can also be prepared by passing fluorine gas over TeO3 or indirectly by reacting TeO2 with SeF4 to produce TeF4 and then heating TeF4 in excess of 200 °C to make TeF6 and Te.
Properties
Tellurium hexafluoride is a highly symmetric octahedral molecule. Its physical properties resemble the sulfur and selenium analogs. It is less volatile, however, due to the increase in polarizability. At temperatures below −38 °C, tellurium hexafluoride condenses to a volatile white solid.
Reactivity
Unlike the sulfur analog, tellurium hexafluoride is not chemically inert. This can be attributed to the larger atomic radius which can co-ordinate a maximum of eight atoms rather than six for sulfur and selenium which allows for nucleophilic attack. TeF6 is hydrolyzed in water to Te(OH)6 and reacts with Te below 200 °C.
- TeF6 + 6 H2O → Te(OH)6 + 6 HF
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0588". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b CRC Handbook of Chemistry and Physics, 90. Auflage, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-95.
- ^ "Tellurium hexafluoride (as Te)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
Literature
- W.C. Cooper; Tellurium, Van Nostrand Reinhold Company, New York, USA, 1971.
- K.W. Bagnall; The Chemistry of Selenium, Tellurium and Polonium, Elsevier Publishing, New York, 1966.
- R.T. Sanderson; Chemical Periodicity, Reinhold, New York, USA, 1960.
- N.N. Greenwood and A. Earnshaw; Chemistry of the Elements, 2nd edition, Butterworth, UK, 1997.
- F. A. Cotton, G. Wilkinson, C.A. Murillo, and M. Bochmann; Advanced Inorganic Chemistry, John Wiley & Sons, 1999.
- G.J. Hathaway, N.H. Proctor; Chemical Hazards of the Workplace, 5th edition, Wiley-Interscience, New Jersey, 2004.