 | |||
| | |||
| Names | |||
|---|---|---|---|
| IUPAC name Butanedinitrile[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| 1098380 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.441 | ||
| EC Number |
| ||
| MeSH | succinonitrile | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | C4H4N2 | ||
| Molar mass | 80.090 g·mol−1 | ||
| Appearance | Colorless, waxy crystals | ||
| Odor | odorless[2] | ||
| Density | 985 mg mL−1 | ||
| Melting point | 58.078 °C; 136.540 °F; 331.228 K | ||
| Boiling point | 266.1 °C; 510.9 °F; 539.2 K | ||
| 130 g L−1 | |||
| Vapor pressure | 300 Pa (at 100 °C) | ||
| Thermochemistry | |||
Heat capacity (C) | 145.60 J K−1 mol−1 | ||
Std molar entropy (S | 191.59 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) | 139.3–140.4 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) | −2.2848–−2.2860 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
GHS hazard statements | H302, H315, H319, H335 | ||
GHS precautionary statements | P261, P305+351+338 | ||
| Flash point | 113 °C (235 °F; 386 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 450 mg kg−1 (oral, rat) | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | none[2] | ||
REL (Recommended) | TWA 6 ppm (20 mg/m3)[2] | ||
IDLH (Immediate danger) | N.D.[2] | ||
| Related compounds | |||
Related alkanenitriles |
| ||
Related compounds | DBNPA | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
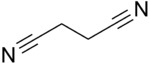
Succinonitrile, also butanedinitrile, is a nitrile, with the formula of C2H4(CN)2. It is a colorless solid that melts at 58[3] °C, hence its waxy consistency.
Succinonitrile is produced by the addition of hydrogen cyanide to acrylonitrile:[4]
- CH2=CHCN + HCN → NCCH2CH2CN
Hydrogenation of succinonitrile yields putrescine (1,4-diaminobutane).
References
- ^ "succinonitrile - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 14 June 2012.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0573". National Institute for Occupational Safety and Health (NIOSH).
- ^ Rubinstein, E. R.; Tirmizi, S. H.; Glicksman, M. E. (1990-11-01). "Long-term purity assessment in succinonitrile". Journal of Crystal Growth. 106 (1): 89–96. doi:10.1016/0022-0248(90)90290-2. ISSN 0022-0248.
- ^ "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry (7th ed.). Retrieved 2007-09-10.
External links