 | |
| Clinical data | |
|---|---|
| Trade names | Rhythmy |
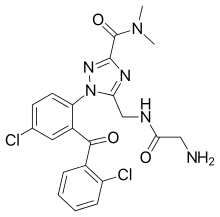
| Other names | 5-([(2-aminoacetyl)amino]methyl)-1-[4-chloro-2-(2-chlorobenzoyl)phenyl]-N,N-dimethyl-1,2,4-triazole-3-carboxamide |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral (tablets) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 10.5 h |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H20Cl2N6O3 |
| Molar mass | 475.33 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Rilmazafone[1] (リスミー, Rhythmy, previously known as 450191-S) is a water-soluble benzodiazepine prodrug developed in Japan.[2] It has sedative and hypnotic effects.[3][4] Rilmazafone induces impairment of motor function and has hypnotic properties.[5]
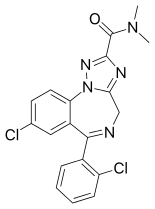
Rilmazafone has no effects on benzodiazepine receptors itself, but once inside the body is metabolised by aminopeptidase enzymes in the small intestine to form the active benzodiazepine 8-chloro-6-(2-chlorophenyl)-N,N-dimethyl-4H-1,2,4-triazolo [1,5-a][1,4]benzodiazepine-2-carboxamide.[6][7]
See also
- Avizafone
- Alprazolam triazolobenzophenone
- GL-II-73
- CCRIS-1930
References
- ^ DE Patent 2725164
- ^ Yamamoto K, Hirose K, Matsushita A, Yoshimura K, Sawada T, Eigyo M, Jyoyama H, Fujita A, Matsubara K, Tsukinoki Y (July 1984). "[Pharmacological studies of a new sleep-inducer, 1H-1,2,4-triazolyl benzophenone derivatives (450191-S) (I). Behavioral analysis]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 84 (1): 109–54. doi:10.1254/fpj.84.109. PMID 6149174.
- ^ Yamamoto K, Matsushita A, Sawada T, Naito Y, Yoshimura K, Takesue H, Utsumi S, Kawasaki K, Hirono S, Koshida H (July 1984). "[Pharmacology of a new sleep inducer, 1H-1,2,4-triazolyl benzophenone derivative, 450191-S (II). Sleep-inducing activity and effect on the motor system]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 84 (1): 25–89. doi:10.1254/fpj.84.25. PMID 6149175.
- ^ Ibii N, Horiuchi M, Yamamoto K (August 1984). "[Pharmacology of a 1H-1, 2, 4-triazolyl benzophenone derivative (450191-S), a new sleep-inducer (III). Behavioral study on interactions of 450191-S and other drugs in mice]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 84 (2): 155–73. doi:10.1254/fpj.84.155. PMID 6149177.
- ^ Yasui M, Kato A, Kanemasa T, Murata S, Nishitomi K, Koike K, et al. (June 2005). "[Pharmacological profiles of benzodiazepinergic hypnotics and correlations with receptor subtypes]". Nihon Shinkei Seishin Yakurigaku Zasshi = Japanese Journal of Psychopharmacology. 25 (3): 143–51. PMID 16045197.
- ^ Koike M, Norikura R, Sugeno K (March 1986). "Intestinal activation of a new sleep inducer 450191-S, a 1H-1,2,4-triazolyl benzophenone derivative, in rats". Journal of Pharmacobio-Dynamics. 9 (3): 315–20. doi:10.1248/bpb1978.9.315. PMID 3454653.
- ^ Muranushi N, Miyauchi S, Suzuki H, Sugiyama Y, Hanano M, Kinoshita H, Oguma T, Yamada H (May 1993). "Comparative hepatic transport of desglycylated and cyclic metabolites of rilmazafone in rats: analysis by multiple indicator dilution method". Biopharmaceutics & Drug Disposition. 14 (4): 279–90. doi:10.1002/bdd.2510140402. PMID 8499579. S2CID 24923818.
External links
- "リスミー'リルマザホン塩酸塩水和物錠 Rhythmy (rilmazafone hydrochloride hydrate, tablets) Prescribing Information" (PDF) (in Japanese). Shionogi & Co., Ltd.
- Drug Information Sheet