| | |||
| Names | |||
|---|---|---|---|
| IUPAC name Potassium hydrogen phthalate | |||
| Other names hydrogen potassium phthalate; phthalic acid potassium salt; potassium biphthalate; potassium acid phthalate; 1,2-benzenedicarboxylic acid, monopotassium salt; KHP; KHPh | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.011.718 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
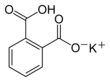
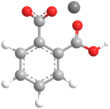
Chemical formula | C8H5KO4 | ||
| Molar mass | 204.222 g·mol−1 | ||
| Appearance | White or colorless solid | ||
| Density | 1.636 g/cm3 | ||
| Melting point | ~295 °C (decomposes) | ||
| 80 g/L (20 °C)[1] | |||
| Solubility | slightly soluble in alcohol | ||
| Acidity (pKa) | 5.4[2] | ||
| Structure | |||
| tetrahedral | |||
| Hazards | |||
| Main hazards | Irritant to eyes, skin, and respiratory system | ||
| Safety data sheet | External MSDS | ||
| R-phrases (outdated) | R36 R37 R38 | ||
| Flash point | Non-flammable | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Potassium hydrogen phthalate, often called simply KHP, is an acidic salt compound. It forms white powder, colorless crystals, a colorless solution, and an ionic solid that is the monopotassium salt of phthalic acid. KHP is slightly acidic, and it is often used as a primary standard for acid-base titrations because it is solid and air-stable, making it easy to weigh accurately. It is not hygroscopic.[3][4][5] It is also used as a primary standard for calibrating pH meters because, besides the properties just mentioned, its pH in solution is very stable. It also serves as a thermal standard in thermogravimetric analysis.[6]
KHP dissociates completely in water, giving the potassium cation (K+) and hydrogen phthalate anion (HP− or Hphthalate−).
- KHP + H2O ⇌ K+ + HP−
And then as a weak acid hydrogen phthalate reacts reversibly with water to give hydronium (H3O+) and phthalate ions.
- HP− + H2O ⇌ P2− + H3O+
KHP can be used as a buffering agent in combination with hydrochloric acid (HCl) or sodium hydroxide (NaOH) depending on which side of pH 4.0 the buffer is to be.
KHP is also a useful standard for total organic carbon (TOC) testing. Most TOC analyzers are based on the oxidation of organics to carbon dioxide and water, with subsequent quantitation of the carbon dioxide. Many TOC analysts suggest testing their instruments with two standards: one typically easy for the instrument to oxidize (KHP), and one more difficult to oxidize. For the latter, benzoquinone is suggested.
References
- ^ http://www.merckmillipore.com/INTL/en/product/pharmaceutical-ingredients/potassium-hydrogen-phthalate,MDA_CHEM-104874
- ^ http://archpdfs.lps.org/Chemicals/Potassium%20Hydrogen%20Phthalate.pdf
- ^ Hendrixson, W. S. (1920). "Further Work on Potassium Hydrogen Phthalate as a Standard in Volumetric Analysis". J Am Chem Soc. 42 (4): 724–727. doi:10.1021/ja01449a008.
- ^ "Potassium Hydrogen Phthalate". Arlington, TX: Ricca Chemical Company. Archived from the original on 2012-11-30. Retrieved 2012-10-03.
- ^ "The Standardization Of NaOH and KHP Assay" (PDF). Clark College. Archived from the original (PDF) on 2012-11-19. Retrieved 2012-10-03.
- ^ Smalley, I.J.,Lill,G.O.,Bentley,S.P.,Wood,D.R. 1977. Thermogravimetry of potassium hydrogen phthalate and its use as a thermal standard. Canadian Mineralogist 15, 30-35