 | |
 | |
| Names | |
|---|---|
| IUPAC name Potassium hydrogen sulfite | |
| Other names Potassium bisulfite, potassium bisulphite, monopotassium salt, monopotassium sulfite, potassium hydrosulfite | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.028.973 |
| EC Number |
|
| E number | E228 (preservatives) |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
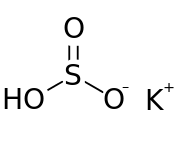
Chemical formula | KHSO3 |
| Molar mass | 120.1561 g/mol |
| Appearance | White crystalline powder |
| Odor | SO2-like |
| Melting point | 190 °C (374 °F; 463 K) (decomposes) |
| 49 g/100 mL (20 °C) 115 g/100 mL (100 °C) | |
| Solubility | Insoluble in alcohol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximate chemical formula KHSO3. Potassium bisulfite in fact is not a real compound,[1] but a mixture of salts that dissolve in water to give solutions composed of potassium ions and bisulfite ions. It is a white solid with an odor of sulfur dioxide. Attempts to crystallize potassium bisulfite yield potassium metabisulfite, K2S2O5.[2]
Potassium bisulfite is used as a sterilising agent in the production of alcoholic beverages.[3] This additive is classified as E number E228 under the current EU-approved food additive legislation.[4]
Production
It is made by the reaction of sulfur dioxide and potassium carbonate. The sulfur dioxide is passed through a solution of the potassium carbonate until no more carbon dioxide is evolved. The solution is concentrated.
See also
- Calcium bisulfite
- Sodium bisulfite
References
- ^ Tudela, David; Jenkins, H. Donald B. (2003). "New Methods to Estimate Lattice Energies: Application to the Relative Stabilities of Bisulfite (HSO3−) and Metabisulfite (S2O52-) Salts". Journal of Chemical Education. 80 (12): 1482. Bibcode:2003JChEd..80.1482T. doi:10.1021/ed080p1482.
- ^ Johnstone, H. F. (1946). "Sulfites and Pyrosulfites of the Alkali Metals". Inorganic Syntheses. Inorganic Syntheses. 2. pp. 162–167. doi:10.1002/9780470132333.ch49. ISBN 9780470132333.
- ^ Barberá, José Jiménez; Metzger, Adolf; Wolf, Manfred (2000). "Sulfites, Thiosulfates, and Dithionitesl Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_477.
- ^ "Approved additives and E numbers". Food Standards Agency. Retrieved 2020-04-07.