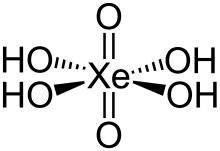
In chemistry, perxenates are salts of the yellow[1] xenon-containing anion XeO4−
6.[2] This anion has octahedral molecular geometry, as determined by Raman spectroscopy, having O–Xe–O bond angles varying between 87° and 93°.[3] The Xe–O bond length was determined by X-ray crystallography to be 1.875 Å.[4]
Synthesis
Perxenates are synthesized by the disproportionation of xenon trioxide when dissolved in strong alkali:[5]
- 2 XeO3 (s) + 4 OH− (aq) → Xe (g) + XeO4−
6 (aq) + O2 (g) + 2 H2O (l)
When Ba(OH)2 is used as the alkali, barium perxenate can be crystallized from the resulting solution.[5]
Perxenic acid
Perxenic acid is the unstable conjugate acid of the perxenate anion, formed by the solution of xenon tetroxide in water. It has not been isolated as a free acid, because under acidic conditions it rapidly decomposes into xenon trioxide and oxygen gas:[6][7]
- 2 HXeO3−
6 + 6 H+
→ 2 XeO
3 + 4 H
2O + O
2
Its extrapolated formula, H4XeO6, is inferred from the octahedral geometry of the perxenate ion (XeO4−
6) in its alkali metal salts.[6][4]
The pKa of aqueous perxenic acid has been indirectly calculated to be below 0, making it an extremely strong acid. Its first ionization yields the anion H
3XeO−
6, which has a pKa value of 4.29, still relatively acidic. The twice deprotonated species H
2XeO2−
6 has a pKa value of 10.81.[8] Due to its rapid decomposition under acidic conditions as described above, however, it is most commonly known as perxenate salts, bearing the anion XeO4−
6.[6][2]
Properties
Perxenic acid and the anion XeO4−
6 are both strong oxidizing agents,[9] capable of oxidising silver(I) to silver(III), copper(II) to copper(III),[10] and manganese(II) to permanganate.[11] The perxenate anion is unstable in acidic solutions,[10] being almost instantaneously reduced to HXeO−
4.[1]
The sodium, potassium, and barium salts are soluble.[12] Barium perxenate solution is used as the starting material for the synthesis of xenon tetroxide (XeO4) by mixing it with concentrated sulfuric acid:[13]
- Ba2XeO6 (s) + 2 H2SO4 (l) → XeO4 (g) + 2 BaSO4 (s) + 2 H2O (l)
Most metal perxenates are stable, except silver perxenate, which decomposes violently.[10]
Applications
Sodium perxenate, Na4XeO6, can be used for the analytic separation of trace amounts of americium from curium. The separation involves the oxidation of Am3+ to Am4+ by sodium perxenate in acidic solution in the presence of La3+, followed by treatment with calcium fluoride, which forms insoluble fluorides with Cm3+ and La3+, but retains Am4+ and Pu4+ in solution as soluble fluorides.[9]
References
- ^ a b Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, p. 593, ISBN 0-471-19957-5
- ^ a b Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 399, ISBN 0-12-352651-5
- ^ Peterson, J. L.; Claassen, H. H.; Appelman, E. H. (March 1970). "Vibrational spectra and structures of xenate(VI) and perxenate(VIII) ions in aqueous solution". Inorganic Chemistry. 9 (3): 619–621. doi:10.1021/ic50085a037.
- ^ a b Hamilton; Ibers, J.; MacKenzie, D. (Aug 1963). "Geometry of the Perxenate Ion". Science. 141 (3580): 532–534. Bibcode:1963Sci...141..532H. doi:10.1126/science.141.3580.532. ISSN 0036-8075. PMID 17738629.
- ^ a b Harding, Charlie; Johnson, David Arthur; Janes, Rob (2002). Elements of the p Block. Molecular World. 9. Royal Society of Chemistry. p. 93. ISBN 0-85404-690-9.
- ^ a b c Klaening, U. K.; Appelman, E. H. (October 1988). "Protolytic properties of perxenic acid". Inorganic Chemistry. 27 (21): 3760–3762. doi:10.1021/ic00294a018.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, p. 400, ISBN 0-12-352651-5
- ^ John H. Holloway; Eric G. Hope (1998). A. G. Sykes (ed.). Advances in Inorganic Chemistry. 46. Academic Press. p. 67. ISBN 0-12-023646-X.
- ^ a b Holcomb, H. P. (March 1965). "Analytical Oxidation of Americium with Sodium Perxenate". Analytical Chemistry. 37 (3): 415. doi:10.1021/ac60222a002.
- ^ a b c Allen J. Bard; Roger Parsons; Joseph Jordan; International Union of Pure and Applied Chemistry (1985). Standard Potentials in Aqueous Solution. CRC Press. p. 778. ISBN 0-8247-7291-1.
- ^ Linus Pauling (1988). General Chemistry (3rd ed.). Courier Dover Publications. p. 251. ISBN 0-486-65622-5.
- ^ Thomas Scott; Mary Eagleson (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 1183. ISBN 3-11-011451-8.
- ^ Charlie Harding; David Arthur Johnson; Rob Janes (2002). Elements of the p block. Great Britain: Royal Society of Chemistry. pp. 92–93. ISBN 0-85404-690-9.