 | |
 | |
| Names | |
|---|---|
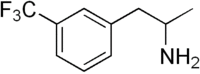
| IUPAC name 1-[3-(Trifluoromethyl)phenyl]propan-2-amine | |
| Other names 3-Trifluoromethylamphetamine | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
IUPHAR/BPS | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | C10H12F3N |
| Molar mass | 203.208 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Norfenfluramine, or 3-trifluoromethylamphetamine, is a never-marketed drug of the amphetamine family that behaves as a serotonin and norepinephrine releasing agent and potent 5-HT2A, 5-HT2B, and 5-HT2C agonist. The action of norfenfluramine on 5-HT2B receptors on heart valves leads to a characteristic pattern of heart failure following proliferation of cardiac fibroblasts on the tricuspid valve, known as cardiac fibrosis.[1] This side effect led to the withdrawal of fenfluramine as an anorectic agent worldwide, and to the withdrawal of benfluorex in Europe,[2] as both fenfluramine and benfluorex form norfenfluramine as an active metabolite. It is a human TAAR1 agonist.[3]
See also
- Fenfluramine
- Benfluorex
- Norfenfluramine is the precursor to flucetorex
References
- ^ Setola, V.; Dukat, M.; Glennon, R. A.; Roth, B. L. (2005). "Molecular Determinants for the Interaction of the Valvulopathic Anorexigen Norfenfluramine with the 5-HT2B Receptor" (PDF). Molecular Pharmacology. 68 (1): 20–33. doi:10.1124/mol.104.009266. PMID 15831837. S2CID 30906680.
- ^ "European Medicines Agency recommends withdrawal of benfluorex from the market in European Union" (PDF). European Medicines Agency. 2009-12-18. Archived from the original (PDF) on 2009-12-22.
- ^ Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–8. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.