 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.073 |
| Chemical and physical data | |
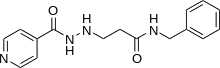
| Formula | C16H18N4O2 |
| Molar mass | 298.346 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| |
| (verify) | |
Nialamide (Niamid, Niamide, Nuredal, Surgex) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class that was used as an antidepressant.[1] It was withdrawn by Pfizer several decades ago due to the risk of hepatotoxicity.[2][3]
The antiatherogenic activity of nialamide was used to design pyridinolcarbamate.[4]
See also
- Hydrazine (antidepressant)
References
- ^ William Andrew Publishing (1 December 2006). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3.
- ^ Gad SC (26 April 2012). Safety Pharmacology in Pharmaceutical Development: Approval and Post Marketing Surveillance, Second Edition. CRC Press. pp. 138–. ISBN 978-1-4398-4567-7.
- ^ Shorter E (28 September 2008). Before Prozac: The Troubled History of Mood Disorders in Psychiatry: The Troubled History of Mood Disorders in Psychiatry. Oxford University Press. pp. 137–. ISBN 978-0-19-970933-5.
- ^ Bencze WL, Hess R, DeStevens G (6 December 2012). "Hypolipidemic agents". Progress in Drug Research. Fortschritte der Arzneimittelforschung. Progrès des Recherches Pharmaceutiques. Springer Science & Business Media. 13: 217–92. doi:10.1007/978-3-0348-7068-9_5. ISBN 9783642661907. PMID 4982663. Retrieved 3 October 2017.