 | |
 | |
| Names | |
|---|---|
| IUPAC name potassium tetraiodidomercurate(II) | |
| Other names potassium mercuric iodide, Nessler's reagent (principal component) | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.082 |
| EC Number |
|
PubChem CID | |
| UNII | |
| UN number | 3287 |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
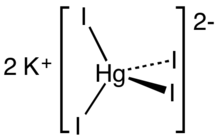
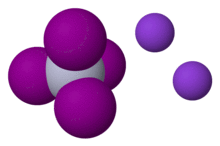
Chemical formula | K2[HgI4][1] |
| Appearance | yellow crystals |
| Odor | odorless |
| Density | 4.29 g/cm3 |
| very soluble | |
| Solubility | soluble in alcohol, ether, acetone |
| Hazards | |
| Safety data sheet | External MSDS for Nessler's reagent |
| Related compounds | |
Other anions | Mercury(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Potassium tetraiodomercurate(II) is an inorganic compound consisting of potassium cations and the tetraiodomercurate(II) anion. It is mainly used as Nessler's reagent, a 0.09 mol/L solution of potassium tetraiodomercurate(II) (K2[HgI4]) in 2.5 mol/L potassium hydroxide, used to detect ammonia.[2]
Preparation and structure
Crystallizing from a concentrated aqueous solution of mercuric iodide with potassium iodide is the monohydrate KHgI3.H2O, which is pale orange.[3] In aqueous solution this triodido complex adds iodide to give the tetrahedral tetraiodo dianion.[4]
Solutions of K2HgI4 react with Cu(I) salts to give Cu2HgI4.[5]
Nessler's reagent
Named after Julius Neßler (Nessler), an alkaline solution of K2HgI4 is called Nessler's reagent. This pale solution becomes deeper yellow in the presence of ammonia. At higher concentrations, a brown precipitate may form. The sensitivity as a spot test is about 0.3 μg NH3 in 2 μL.
- NH4+ + 2 [HgI4]2− + 4 OH− → + 7 I− + 3 H2O
The formula for the brown precipitate, a derivative of Millon's base, is given as 3HgO·Hg(NH3)2I2 and as NH2·Hg2I3.[6]
References
- ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. p. 4-82. ISBN 978-1-4200-9084-0.
- ^ Vogel, Arthur I.; Svehla, G. (1979), Vogel's Textbook of Macro and Semimicro Qualitative Inorganic Analysis (5th ed.), London: Longman, ISBN 0-582-44367-9
- ^ F. Wagenknecht, R. Juza, "Potassium Triiodomercurate(II)" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1100.
- ^ Mok, K. F.; McKee, V. (1990). "Structure of a dipotassium tetraiodomercurate(II) salt with dibenzo-18-crown-6". Acta Crystallographica C. 46 (11): 2078–2081. doi:10.1107/S0108270190003742.
- ^ F. Wagenknecht, R. Juza, "Copper(I) Tetraiodomercurate(II)" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1100.
- ^ Svehla, G. (1979). Vogel's Textbook of Macro and semimicro qualitative inorganic analysis (5th ed.). London: Longman Group. pp. 293–294. ISBN 0-582-44367-9.