 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.462 |
| Chemical and physical data | |
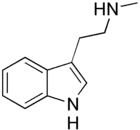
| Formula | C11H14N2 |
| Molar mass | 174.247 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 87 to 89 °C (189 to 192 °F) |
SMILES
| |
InChI
| |
| (verify) | |
N-Methyltryptamine (NMT) is a member of the substituted tryptamine chemical class and a natural product which is biosynthesized in the human body from tryptamine by certain N-methyltransferase enzymes, such as indolethylamine N-methyltransferase.[1][2] It is a common component in human urine.[3] NMT is an alkaloid derived from L-tryptophan that has been found in the bark, shoots and leaves of several plant genera, including Virola, Acacia, Mimosa, and Desmanthus—often together with the related compounds N,N-dimethyltryptamine (DMT) and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT).
Orally administered NMT appears to produce no psychoactive effects, likely as a result of extensive first-pass metabolism.[4] However, it may become active upon combination with a MAOA inhibitor (MAOI).[4] By vaporization NMT shows activity at 50–100 mg, with a duration of 45–70 minutes; duration of visual effects 15–30 seconds. Effects are primarily non-visual.[5][6]
See also
- N-Ethyltryptamine (NET)
- N,N,-Dimethyltryptamine (DMT)
- Acacia confusa (a natural source of NMT, with other tryptamines, 1.63%. Buchanan et al. 2007)
- Acacia obtusifolia (NMT up to 2/3 alkaloid content)
- Acacia simplicifolia (synon. A. simplex) (1.44% NMT in bark, 0.29% twigs, Pouet et al. 1976)
- Desmanthus Illinoensis (NMT major component seasonally)
References
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Burchett SA, Hicks TP (August 2006). "The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain". Prog. Neurobiol. 79 (5–6): 223–46. doi:10.1016/j.pneurobio.2006.07.003. PMID 16962229. S2CID 10272684.
- ^ Scand J Clin Lab Invest. 2001;61(7):547-56. Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS. Forsström T, Tuominen J, Karkkäinen J.
- ^ a b Foye's principles of medicinal chemistry By William O. Foye, Thomas L. Lemke, David A. Williams
- ^ Shulgin & Shulgin "TIKHAL" 1997
- ^ Nen - lecture presented EGA conference, Victoria, Australia 4/12/2011; and Breaking Conventions, London 12/7/2013.