 | |
 | |
| Names | |
|---|---|
| IUPAC name Aminomethanol | |
| Other names Methanolamine | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | CH5NO |
| Molar mass | 47.057 g·mol−1 |
| Appearance | Colorless liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
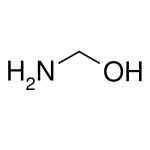
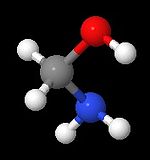
Aminomethanol (also called "methanolamine")[1] is the simplest aminoalcohol and has the chemical formula of CH5NO. It contains both a primary amine and a primary alcohol. With both groups at the same carbon atom, it is also the simplest hemiaminal (derived from formaldehyde and ammonia).[2] Like other amines, methanolamine acts as a weak base.[3]
In aqueous solution, methanolamine may decompose into formaldehyde and ammonia.[4]
See also
- Methanol
- Methylamine
- Ethanolamine (2-aminoethanol)
References
- ^ Pubchem. "Methanolamine". pubchem.ncbi.nlm.nih.gov. Retrieved 2019-01-05.
- ^ Berski, Sławomir; Gordon, Agnieszka J.; Ćmikiewicz, Agnieszka (2018-02-01). "Characterisation of the reaction mechanism between ammonia and formaldehyde from the topological analysis of ELF and catastrophe theory perspective". Structural Chemistry. 29 (1): 243–255. doi:10.1007/s11224-017-1024-x. ISSN 1572-9001. S2CID 103059739.
- ^ Chan, W.; White, Peter (2000). Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford, New York: Oxford University Press. p. 247. ISBN 9780199637249.
- ^ T Feldmann, Michael; Widicus Weaver, Susanna; Blake, Geoffrey; R Kent, David; Goddard, William (2005-08-01). "Aminomethanol water elimination: Theoretical examination". The Journal of Chemical Physics. 123 (3): 34304. Bibcode:2005JChPh.123c4304F. doi:10.1063/1.1935510. PMID 16080734.