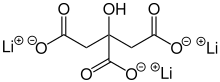
 | |
| Names | |
|---|---|
| Other names Trilithium citrate trilithium 2-hydroxypropane-1,2,3-tricarboxylate | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.860 |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | Li3C6H5O7 |
| Molar mass | 209.923 g mol−1 |
| Appearance | Odorless white powder |
| Melting point | decomposes at 105 °C (221 °F; 378 K) |
| Hazards | |
| Main hazards | Toxic |
| R-phrases (outdated) | R22 R36 R37 R38 |
| Flash point | N/A |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Lithium citrate (Li3C6H5O7) is a chemical compound of lithium and citrate that is used as a mood stabilizer in psychiatric treatment of manic states and bipolar disorder.[1][2] There is extensive pharmacology of lithium, the active component of this salt.
Lithia water contains various lithium salts, including the citrate. An early version of Coca-Cola available in pharmacies' soda fountains called Lithia Coke was a mixture of Coca-Cola syrup and lithia water. The soft drink 7Up was originally named "Bib-Label Lithiated Lemon-Lime Soda" when it was formulated in 1929 because it contained lithium citrate. The beverage was a patent medicine marketed as a cure for hangover. Lithium citrate was removed from 7Up in 1948.[3]
References
- ^ Medication description
- ^ Medical use Archived 2006-06-15 at the Wayback Machine
- ^ Gielen, Marcel; Edward R. T. Tiekink (2005). Metallotherapeutic drugs and metal-based diagnostic agents: The use of metals in medicine. John Wiley and Sons. p. 3. ISBN 0-470-86403-6.