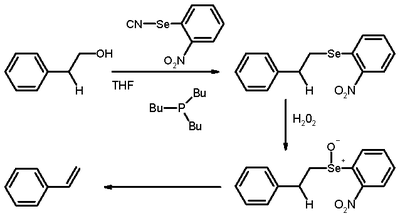
The Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene.[1][2] It is named for Paul Grieco.
The alcohol first reacts with o-nitrophenylselenocyanate and tributylphosphine to form a selenide via a nucleophilic substitution on the electron-deficient selenium. In the second step, the selenide is oxidized with hydrogen peroxide to give a selenoxide. This structure decomposes to form an alkene by an Ei elimination mechanism with expulsion of a selenol in a fashion similar to that of the Cope elimination. This reaction takes part in the synthesis of ring C of the Danishefsky Taxol synthesis.
References
- ^ Organoselenium chemistry. A facile one-step synthesis of alkyl aryl selenides from alcohols Paul A. Grieco, Sydney Gilman, Mugio Nishizawa; J. Org. Chem.; 1976; 41(8); 1485-1486. doi:10.1021/jo00870a052
- ^ Olefin synthesis. Rate enhancement of the elimination of alkyl aryl selenoxides by electron-withdrawing substituents K. Barry Sharpless and Michael W. Young J. Org. Chem.; 1975; ; 947 - 949. doi:10.1021/jo00895a030