| | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 5-Methylidenecyclopenta-1,3-diene[1] | |||
| Other names Fulvene[1] 5-Methylene-1,3-cyclopentadiene | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
SMILES
| |||
| Properties | |||
Chemical formula | C6H6 | ||
| Molar mass | 78.114 g·mol−1 | ||
| -42.9·10−6 cm3/mol | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
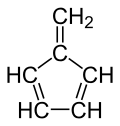
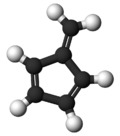
Fulvene(Pentafulvene) usually refers to the hydrocarbon (CH=CH)2C=CH2. It is a prototype of a cross-conjugated hydrocarbon.[2] The parent, fulvene itself, is rarely encountered,[3] but substituted derivatives are numerous. They are mainly of interest as ligands and precursors to ligands in organometallic chemistry. They are often yellow.
Preparation
Substituted fulvenes are readily prepared by the condensation of cyclopentadiene and aldehydes and ketones:
- C5H6 + R2C=O → C4H4C=CR2 + H2O
Thiele is credited with discovering this reaction.[4][5]
Modern synthesis of fulvenes employ buffer systems.[6][7]
Fulvenes
Several types of fulvenes are defined.[8] They are:
- pentafulvene
- triafulvene
- heptafulvene
- nonafulvene
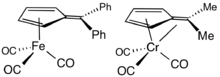
Ligand in organometallic chemistry
Fulvenes are common ligands and ligand precursors in organometallic chemistry.[9] 2,3,4,5-Tetramethylfulvene, abbreviated Me4Fv, results from the deprotonation of cationic pentamethylcyclopentadienyl complexes.[10] Some Me4Fv complexes are called tuck-in complexes.
See also
References
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 379. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Preethanuj Preethalayam; Syam krishnan, K.; Sreeja Thulasi; Sarath Chand,S.; Jomy Joseph; Vijay Nair; Florian Jaroschik; K.V.Radhakrishnan (2017). "Recent Advances in the Chemistry of Pentafulvenes". Chemical Reviews. 117 (5): 3930–3989. doi:10.1021/acs.chemrev.6b00210. PMID 28151643.
- ^ Bergmann, E. D. (1968). "Fulvenes and Substituted Fulvenes". Chemical Reviews. 68: 41–84. doi:10.1021/cr60251a002.
- ^ Thiele, J. (1900). "Ueber Ketonreactionen bei dem Cyclopentadiën". Chemische Berichte. 33: 666–673. doi:10.1002/cber.190003301113.
- ^ Hafner, K.; Vöpel, K. H.; Ploss, G.; König, C. (1967). "6-(Dimethylamino)Fulvene". Organic Syntheses. 47: 52. doi:10.15227/orgsyn.047.0052.
- ^ Coşkun, Necdet; Erden, Ihsan (2011-11-11). "An efficient catalytic method for fulvene synthesis". Tetrahedron. 67 (45): 8607–8614. doi:10.1016/j.tet.2011.09.036. ISSN 0040-4020. PMC 3196336. PMID 22021940.
- ^ Sieverding, Paul; Osterbrink, Johanna; Besson, Claire; Kögerler, Paul (2019-01-18). "Kinetics and mechanism of pyrrolidine buffer-catalyzed fulvene formation". J. Org. Chem. 84 (2): 486–494. doi:10.1021/acs.joc.8b01660. ISSN 0022-3263. PMID 30540466.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Fulvenes". doi:10.1351/goldbook.F02550
- ^ Strohfeldt, Katja; Tacke, Matthias (2008). "Bioorganometallic fulvene-derived titanocene anti-cancer drugs". Chemical Society Reviews. 37 (6): 1174–87. doi:10.1039/B707310K. PMID 18497930.
- ^ Kreindlin, A. Z.; Rybinskaya, M. A. (2004). "Cationic and Neutral Transition Metal Complexes with a Tetramethylfulvene or Trimethylallyldiene Ligand". Russian Chemical Reviews. 73 (5): 417–432. Bibcode:2004RuCRv..73..417K. doi:10.1070/RC2004v073n05ABEH000842.