A 4-center 2-electron (4c–2e) bond is a type of chemical bond in which four atoms share two electrons in bonding, with a net bond order of 1&fras1;2. This type of bonding differs from the usual covalent bond, which involves two atoms sharing two electrons (2c–2e bonding).
Four-center two-electron bonding is postulated in certain cluster compounds. For instance, the borane B
6H−
7 anion, is a B
6H2−
6 octahedron with an additional proton attached to one of the triangular faces.[1] As a result, the octahedron is distorted and a B–B–B–H rhomboid ring can be identified in which this 4c–2e bonding takes place. This type of bonding is associated with electron deficient rhomboid rings in general[2] and is a relatively new research field, fitting in with the already well established three-center two-electron bond.
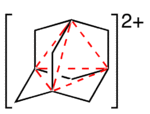
An example of a purely organic compound with four-center two-electron bonding is the adamantyl dication.[3] The bond joins the four bridgehead atoms in a tetrahedral geometry.
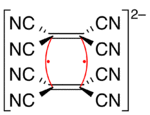
Tetracyanoethylene forms a dianionic dimer in which the two alkenes are joined face-to-face by a rectangular four-center two-electron bond.[4] Various solid salts of this dianion have been studied to determine bond strengths and vibrational spectroscopic details.[5]
References
- ^ Hofmann, K.; Prosenc, M. H.; Albert, B. R. (2007). "A new 4c–2e bond in B
6H−
7". Chem. Commun. 2007: 3097–3099. doi:10.1039/b704944g. - ^ Balakrishnarajan, M. M.; Hoffmann, R. (2004). "Electron-Deficient Bonding in Rhomboid Rings". J. Am. Chem. Soc. 126 (40): 13119–13131. doi:10.1021/ja0467420.
- ^ Bremer, Matthias; Schleyer, Paul von Ragué; Schötz, Karl; Kausch, Michael; Schindler, Michael (1987). "Four-Center Two-Electron Bonding in a Tetrahedral Topology. Experimental Realization of Three-Dimensional Homoaromaticity in the 1,3-Dehydro-5,7-adamantanediyl Dication". Angew. Chem. Int. Ed. Engl. 26: 761–763. doi:10.1002/anie.198707611.
- ^ Del Sesto, R. E.; Miller, J. S.; Lafuente, P.; Novoa, J. J. (2002). "Exceptionally Long (≥2.9 Å) CC Bonding Interactions in π-[TCNE]2−
2 Dimers: Two-Electron Four-Center Cation-Mediated CC Bonding Interactions Involving π* Electrons". Chemistry. 8 (21): 4894–4908. doi:10.1002/1521-3765(20021104)8:21<4894::AID-CHEM4894>3.0.CO;2-2. PMID 12397591. - ^ Casado, J.; Burrezo, P. M.; Ramírez, F. J.; Navarrete, J. T. L.; Lapidus, S. H.; Stephens, P. W.; Vo, H.-L.; Miller, J. S.; Mota, F.; Novoa, J. J. (2013). "Evidence for Multicenter Bonding in Dianionic Tetracyanoethylene Dimers by Raman Spectroscopy". Angew. Chem. Int. Ed. 52 (25): 6421–6425. doi:10.1002/anie.201207813.