| |||
| Names | |||
|---|---|---|---|
| Other names FITC 5-Isomer:
| |||
| Identifiers | |||
| |||
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.043.810 | ||
| MeSH | Fluorescein+isothiocyanate | ||
PubChem CID | |||
| UNII |
| ||
CompTox Dashboard (EPA) | |||
| |||
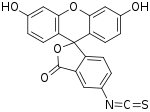
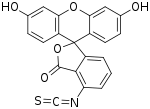
SMILES
| |||
| Properties | |||
| C21H11NO5S | |||
| Molar mass | 389.38 g·mol−1 | ||
| Density | 1.542 g/mL | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Fluorescein isothiocyanate (FITC) is a derivative of fluorescein used in wide-ranging applications[1][2] including flow cytometry. First described in 1942,[3] FITC is the original fluorescein molecule functionalized with an isothiocyanate reactive group (-N=C=S), replacing a hydrogen atom on the bottom ring of the structure. It is typically available as a mixture of isomers, fluorescein 5-isothiocyanate (5-FITC) and fluorescein 6-isothiocyanate (6-FITC). FITC is reactive towards nucleophiles including amine and sulfhydryl groups on proteins. It was synthesized by Robert Seiwald and Joseph Burckhalter in 1958. [4]
A succinimidyl-ester functional group attached to the fluorescein core, creating "NHS-fluorescein", forms another common amine reactive derivative that has much greater specificity toward primary amines in the presence of other nucleophiles.
FITC has excitation and emission spectrum peak wavelengths of approximately 495 nm/519 nm,[5] giving it a green color. Like most fluorochromes, it is prone to photobleaching. Because of the problem with photobleaching, derivatives of fluorescein such as Alexa 488 and DyLight 488 have been tailored for various chemical and biological applications where greater photostability, higher fluorescence intensity, or different attachment groups are needed.
References
- ^ The TH; Feltkamp, T. E. (1970). "Conjugation of fluorescein isothiocyanate to antibodies: I. Experiments on the conditions of conjugation". Immunology. 18 (6): 865–873. PMC 1455721. PMID 5310665.
- ^ The TH; Feltkamp, T. E. (1970). "Conjugation of fluorescein isothiocyanate to antibodies: II. A reproducible method". Immunology. 18 (6): 875–881. PMC 1455722. PMID 4913804.
- ^ Coons AH, Creech HJ, Norman Jones R, Berliner E (1942). "The Demonstration of Pneumococcal Antigen in Tissues by the Use of Fluorescent Antibody". The Journal of Immunology. 45 (3): 159–170.
- ^ Riggs JL, Seiwald RJ, Burckhalter JH (1958). "Isothiocyanate Compounds as Fluorescent Labeling Agents for Immune Serum". The American Journal of Pathology. 34 (6): 1081–1097. PMC 1934794. PMID 13583098.
- ^ "FITC".