| F-box linker domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
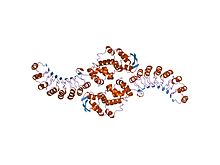 Structure of the LRR linker domain of Skp2 in the Skp1-Skp2 complex.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | F-box | ||||||||
| Pfam | PF00646 | ||||||||
| Pfam clan | CL0271 | ||||||||
| InterPro | IPR001810 | ||||||||
| SMART | SM00256 | ||||||||
| PROSITE | PS50181 | ||||||||
| SCOP2 | 1fs2 / SCOPe / SUPFAM | ||||||||
| Membranome | 630 | ||||||||
| |||||||||
F-box proteins are proteins containing at least one F-box domain. The first identified F-box protein is one of three components of the SCF complex, which mediates ubiquitination of proteins targeted for degradation by the 26S proteasome.
Core components
F-box domain is a protein structural motif of about 50 amino acids that mediates protein–protein interactions. It has consensus sequence and varies in few positions. It was first identified in cyclin F.[2] The F-box motif of Skp2, consisting of three alpha-helices, interacts directly with the SCF protein Skp1.[3] F-box domains commonly exist in proteins in concert with other protein–protein interaction motifs such as leucine-rich repeats (illustrated in the Figure) and WD repeats, which are thought to mediate interactions with SCF substrates.[4]
Function
F-box proteins have also been associated with cellular functions such as signal transduction and regulation of the cell cycle.[5] In plants, many F-box proteins are represented in gene networks broadly regulated by microRNA-mediated gene silencing via RNA interference.[6] F-box proteins are involved in many plant vegetative and reproduction growth and development. For example, F-box protein-FOA1 involved in abscisic acid (ABA) signaling to affect the seed germination.[7] ACRE189/ACIF1 can regulate cell death and defense when the pathogen is recognized in the Tobacco and Tomato plant.[8]
In human cells, under high-iron conditions, two iron atoms stabilise the F-Box FBXL5 and then the complex mediates the ubiquitination of IRP2.[9]
Regulation
F-box protein levels can be regulated by different mechanisms. The regulation can occur via protein degradation process and association with SCF complex . For example, in yeast, the F-box protein Met30 can be ubiquitinated in a cullin-dependent manner.[10][11]
References
- ^ Schulman BA, Carrano AC, Jeffrey PD, et al. (November 2000). "Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex". Nature. 408 (6810): 381–6. Bibcode:2000Natur.408..381S. doi:10.1038/35042620. PMID 11099048. S2CID 4300503.
- ^ Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. "SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box". Cell 86 263-74 1996.
- ^ Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ (July 1996). "SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box". Cell. 86 (2): 263–74. doi:10.1016/S0092-8674(00)80098-7. PMID 8706131.
- ^ Kipreos ET, Pagano M (2000). "The F-box protein family". Genome Biol. 1 (5): REVIEWS3002. doi:10.1186/gb-2000-1-5-reviews3002. PMC 138887. PMID 11178263.
- ^ Craig KL, Tyers M (1999). "The F-box: a new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction". Prog. Biophys. Mol. Biol. 72 (3): 299–328. doi:10.1016/S0079-6107(99)00010-3. PMID 10581972.
- ^ Jones-Rhoades MW, Bartel DP, Bartel B (2006). "MicroRNAS and their regulatory roles in plants". Annu Rev Plant Biol. 57: 19–53. doi:10.1146/annurev.arplant.57.032905.105218. PMID 16669754.
- ^ Peng, Juan; Yu, Dashi; Wang, Liqun; Xie, Minmin; Yuan, Congying; Wang, Yu; Tang, Dongying; Zhao, Xiaoying; Liu, Xuanming (June 2012). "Arabidopsis F-box gene FOA1 involved in ABA signaling". Science China. Life Sciences. 55 (6): 497–506. doi:10.1007/s11427-012-4332-9. ISSN 1869-1889. PMID 22744179.
- ^ Ha, Van Den Burg; Tsitsigiannis, D. I.; Rowland, O; Lo, J; Rallapalli, G; Maclean, D; Takken, F. L.; Jones, J. D. (2008). "The F-box protein ACRE189/ACIF1 regulates cell death and defense responses activated during pathogen recognition in tobacco and tomato". Plant Cell. 20 (3): 697.
- ^ Moroishi, T; Nishiyama, M; Takeda, Y; Iwai, K; Nakayama, K. I. (2011). "The FBXL5-IRP2 axis is integral to control of iron metabolism in vivo". Cell Metabolism. 14 (3): 339.
- ^ Kaiser, Peter; Su, Ning-Yuan; Yen, James L.; Ouni, Ikram; Flick, Karin (2006-08-08). "The yeast ubiquitin ligase SCFMet30: connecting environmental and intracellular conditions to cell division". Cell Division. 1: 16. doi:10.1186/1747-1028-1-16. ISSN 1747-1028.
Further reading
- Ho M, Tsai P, Chien C (2006). "F-box proteins: the key to protein degradation". J Biomed Sci. 13 (2): 181–91. doi:10.1007/s11373-005-9058-2. PMID 16463014.
External links
- F-Box+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
- F-box+motifs at the US National Library of Medicine Medical Subject Headings (MeSH)