 | |
 | |
| Names | |
|---|---|
| IUPAC name 2-(Methacryloyloxy)ethyl methacrylate | |
| Preferred IUPAC name ethane-1,2-diyl bis(2-methylprop-2-enoate) | |
| Other names Methacrylic acid, ethylene ester; 1,2-Bis(methacryloyloxy)ethane; 1,2-Ethanediol dimethacrylate; Diglycol dimethacrylate; Ethanediol dimethacrylate; Ethylene dimethacrylate; Ethylene glycol bis(methacrylate); Ethylene glycol dimethacrylate; Ethylene methacrylate | |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | EGDMA |
| 1776663 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.380 |
| EC Number |
|
Gmelin Reference | 637376 |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
Chemical formula | C10H14O4 |
| Molar mass | 198.218 g·mol−1 |
| Density | 1.051 g/mL |
| Melting point | −40 °C (−40 °F; 233 K) |
| Boiling point | 98 to 100 °C (208 to 212 °F; 371 to 373 K) (5 mmHg) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements | H315, H317, H319, H335, H412 |
GHS precautionary statements | P261, P264, P271, P272, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 |
| Flash point | 101 °C (214 °F; 374 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
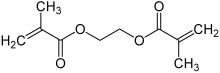
Ethylene glycol dimethylacrylate (EGDMA) is a diester formed by condensation of two equivalents of methacrylic acid and one equivalent of ethylene glycol.[2]
EGDMA can be used in free radical copolymer crosslinking reactions. When used with methyl methacrylate, it leads to gel point at relatively low concentrations because of the nearly equivalent reactivities of all the double bonds involved.
It is used as a monomer to prepare Hydroxyapatite/Poly methyl methacrylate composites. EGDMA can be used in free radical copolymer crosslinking reactions.
References
- ^ Ethylene glycol dimethacrylate at Sigma-Aldrich
- ^ Bielstein 2, IV, 1532