 | |
 | |
| Names | |
|---|---|
| Other names Nitrous anhydride, nitrogen sesquioxide | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.031.013 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| N2O3 | |
| Molar mass | 76.01 g/mol |
| Appearance | deep blue tinted liquid |
| Density | 1.447 g/cm3, liquid 1.783 g/cm3 (gas) |
| Melting point | −100.7[1] °C (−149.3 °F; 172.5 K) |
| Boiling point | 3.5 °C (38.3 °F; 276.6 K)(dissociates[1]) |
| very soluble | |
| Solubility | soluble in ether |
| −16.0·10−6 cm3/mol | |
| Structure | |
| planar, Cs | |
| 2.122 D | |
| Thermochemistry | |
Heat capacity (C) | 65.3 J/mol K |
Std molar entropy (S | 314.63 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) | +91.20 kJ/mol |
| Hazards | |
EU classification (DSD) (outdated) | Highly toxic (T+) |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
| Nitrous oxide Nitric oxide Nitrogen dioxide Dinitrogen tetroxide Dinitrogen pentoxide Nitrogen trioxide | |
Related compounds | Nitrous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dinitrogen trioxide is the chemical compound with the formula N2O3. This deep blue solid[1] is one of the simple nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):[2]
- NO + NO2 ⇌ N2O3
Dinitrogen trioxide is only isolable at low temperatures, i.e. in the liquid and solid phases. At higher temperatures the equilibrium favors the constituent gases, with Kdiss = 193 kPa (25 °C).[3]
This compound is sometimes called "nitrogen trioxide", but this name properly refers to another compound, the (uncharged) nitrate radical NO
3
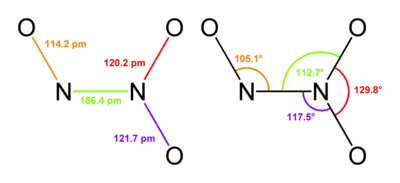
Structure and bonding
Typically, N–N bonds are similar in length to that in hydrazine (145 pm). Dinitrogen trioxide, however, has an unusually long N–N bond at 186 pm. Some other nitrogen oxides also possess long N–N bonds, including dinitrogen tetroxide (175 pm). The N2O3 molecule is planar and exhibits Cs symmetry. The dimensions displayed below come from microwave spectroscopy of low-temperature, gaseous N2O3:[2]
It is the anhydride of the unstable nitrous acid (HNO2), and produces it when mixed into water. An alternative structure might be anticipated for the true anhydride, i.e. O=N–O–N=O, but this isomer is not observed. If the nitrous acid is not then used up quickly, it decomposes into nitric oxide and nitric acid. Nitrite salts are sometimes produced by adding N2O3 to solutions of bases:
- N2O3 + 2 NaOH → 2 NaNO2 + H2O
References
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 444. ISBN 978-0-08-037941-8.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 521–22. ISBN 978-0-08-022057-4.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5