 | |
| Names | |
|---|---|
| Other names 2,3-Dihydroporphine | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C20H16N4 | |
| Molar mass | 312.36784 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
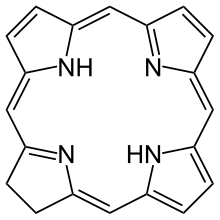
In organic chemistry, a chlorin is a tetrapyrrole pigment, simply these are partially hydrogenated porphyrins.[1] The parent chlorin is an unstable compound which undergoes air oxidation to porphine.
The name chlorin derived from chlorophylls. Chlorophylls are Magnesium containing chlorins, a photosynthetic pigment present in chloroplasts. The reduced chlorin variants are present in bacteriochlorophylls and they are named as bacteriochlorins and isobacteriochlorins.
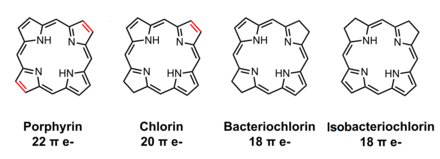
Chlorins are excellent photosensitizing agents. Various synthetic chlorins analogues such as m-tetrahydroxyphenylchlorin (mTHPC) and mono-L-aspartyl chlorin e6 are effectively employed in experimental photodynamic therapy as photosensitizer.[2]
Natural Chlorins
Chlorophyll
The most abundant chlorin is Photosynthetic pigment Chlorophyll. There are different types of chlorophylls, such as Chlorophyll a, Chlorophyll b, Chlorophyll d, Chlorophyll e, Chlorophyll f, Chlorophyll g. The basic unit of these chlorophylls is a chlorin with Magnesium as a central metal atom. [3]
See also
Further reading
- Juse´lius, Jonas; Sundholm, Dage (2000). "The aromatic pathways of porphins, chlorins and bacteriochlorins". Physical Chemistry Chemical Physics. 2 (10): 2145–2151. Bibcode:2000PCCP....2.2145J. doi:10.1039/b000260g.
References
- ^ Gerard P. Moss (1988). "Nomenclature of Tetrapyrroles. Recommendations 1986". European Journal of Biochemistry. 178 (2): 277–328. doi:10.1111/j.1432-1033.1988.tb14453.x. PMID 3208761.
- ^ Spikes, John D. (July 1990). "New trends in photobiology". Journal of Photochemistry and Photobiology B: Biology. 6 (3): 259–274. doi:10.1016/1011-1344(90)85096-F. PMID 2120404.
- ^ K. Eszter, Borbas. Handbook of Porphyrin Science: 181: Chlorins. worldscientific. doi:10.1142/9789813149564_0001. ISBN 9814322326.