 | |
| Clinical data | |
|---|---|
| Trade names | Gynazole-1, Mycelex-3 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682012 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Vaginal cream |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H17Cl3N2S |
| Molar mass | 411.77 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| |
| (verify) | |
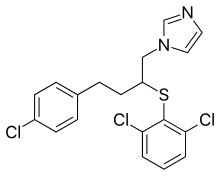
Butoconazole (trade names Gynazole-1, Mycelex-3) is an imidazole antifungal used in gynecology. It is administered as a vaginal cream.[1][2]
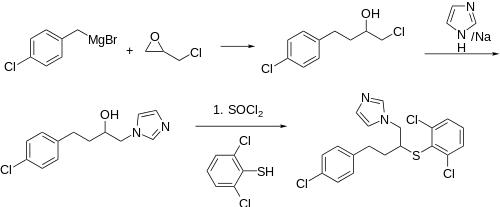
Synthesis
Reaction of epichlorohydrin with 4-Chlorobenzyl magnesium bromide leads to 1-chloro-4-(4-chlorophenyl)butan-2-ol (3). Displacement with sodium imidazole, conversion of the secondary alcohol to the chloride (SOCl2), and displacement with 2,6-dichlorobenzenethiol concludes the synthesis of the antifungal butoconazole.
References
- ^ Seidman LS, Skokos CK (December 2005). "An evaluation of butoconazole nitrate 2% site release vaginal cream (Gynazole-1) compared to fluconazole 150 mg tablets (Diflucan) in the time to relief of symptoms in patients with vulvovaginal candidiasis". Infectious Diseases in Obstetrics and Gynecology. 13 (4): 197–206. doi:10.1155/2005/453239. PMC 1784583. PMID 16338779.
- ^ Butoconazole Monograph
- ^ Walker KA, Braemer AC, Hitt S, Jones RE, Matthews TR (August 1978). "1-[4-(4-Chlorophenyl)-2-(2,6-dichlorophenylthio)-n-butyl]-1H-imidazole nitrate, a new potent antifungal agent". Journal of Medicinal Chemistry. 21 (8): 840–3. doi:10.1021/jm00206a028. PMID 357722.
- ^ US 4078071, Walker KA, "Derivatives of substituted N-alkyl imidazoles", issued 7 March 1978, assigned to Syntex
External links
- "Butoconazole". Drug Information Portal. U.S. National Library of Medicine.