 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.040.180 |
| EC Number |
|
Gmelin Reference | 13627 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| BaS | |
| Molar mass | 169.39 g/mol |
| Appearance | white solid |
| Density | 4.25 g/cm3 [1] |
| Melting point | 2,235[2] °C (4,055 °F; 2,508 K) |
| Boiling point | decomposes |
| 2.88 g/100 mL (0 °C) 7.68 g/100 mL (20 °C) 60.3 g/100 mL (100 °C) | |
| Solubility | insoluble in alcohol |
Refractive index (nD) | 2.155 |
| Structure | |
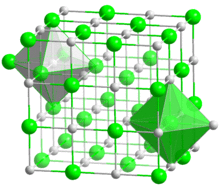
| Halite (cubic), cF8 | |
| Fm3m, No. 225 | |
| Octahedral (Ba2+); octahedral (S2−) | |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements | H315, H319, H335, H400 |
GHS precautionary statements | P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P391, P403+233, P405, P501 |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 226 mg/kg humans |
| Related compounds | |
Other anions | Barium oxide |
Other cations | Magnesium sulfide Calcium sulfide Strontium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Barium sulfide is the inorganic compound with the formula BaS. This colorless salt is an important precursor to other barium compounds including BaCO3 and the pigment lithopone, ZnS/BaSO4.[3] Like other chalcogenides of the alkaline earth metals, BaS is a short wavelength emitter for electronic displays.[4] It is colorless, although like many sulfides, it is commonly obtained in impure colored forms.
Discovery, production and properties
BaS was prepared by Vincentius (or Vincentinus) Casciarolus (or Casciorolus, 1571-1624) via reduction of BaSO4 (available as the mineral barite).[5] It is currently manufactured by an improved version of Casciarolus's process using coke in place of flour. This kind of conversion is called a carbothermic reaction:
- BaSO4 + 2 C → BaS + 2 CO2
The basic methodology remains in use today. BaS dissolves in water. These aqueous solutions, when treated with sodium carbonate or carbon dioxide, give a white solid of barium carbonate, a source material for many commercial barium compounds.[6]
The phosphorescence of the material made by Casciarolus made it a curiosity.[7][8][9]
BaS crystallizes with the NaCl structure, featuring octahedral Ba2+ and S2− centres.
The observed melting point of barium sulfide is highly sensitive to impurities.[2]
Safety
BaS is quite poisonous, as are related sulfides, such as CaS, which evolve toxic hydrogen sulfide upon contact with water.
References
- ^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ a b Stinn, C., Nose, K., Okabe, T. et al. Metall and Materi Trans B (2017) 48: 2922. https://doi.org/10.1007/s11663-017-1107-5
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Vij, D. R.; Singh, N. "Optical and electrical properties of II-VI wide gap semiconducting barium sulfide" Proceedings of SPIE (1992), 1523 (Conf. Phys. Technol. Semicond. Devices Integr. Circuits, 1992), 608-12.
- ^ F. Licetus, Litheosphorus, sive de lapide Bononiensi lucem in se conceptam ab ambiente claro mox in tenebris mire conservante, Utini, ex typ. N. Schiratti, 1640. See http://www.chem.leeds.ac.uk/delights/texts/Demonstration_21.htm Archived 2011-08-13 at the Wayback Machine
- ^ Kresse, Robert; Baudis, Ulrich; Jäger, Paul; Riechers, H. Hermann; Wagner, Heinz; Winkler, Jochen; Wolf, Hans Uwe (2007). "Barium and Barium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_325.pub2.
- ^ "Lapis Boloniensis". www.zeno.org.
- ^ Lemery, Nicolas (1714). Trait℗e universel des drogues simples.
- ^ Ozanam, Jacques; Montucla, Jean Etienne; Hutton, Charles (1814). Recreations in mathematics and natural philosophy .
