 | |
 | |
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| CH4N2O | |
| Molar mass | 60.056 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
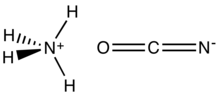
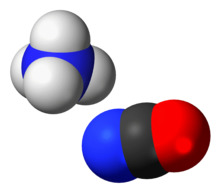
Ammonium cyanate is an inorganic compound with the formula NH4OCN. It is a colorless solid.
Structure and reactions
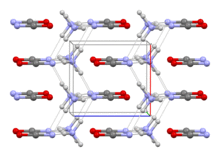
The structure of this salt was verified by X-ray crystallography. The respective C−O and C−N distances are 1.174(8) and 1.192(7) Å, consistent with the O=C=N− description. NH4+ forms hydrogen bonds to N, but not O.[1]
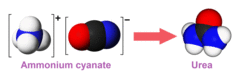
The compound is notable as the precursor in the Wöhler synthesis of urea, an organic compound, from inorganic reactants.[2] This led to the discarding of the Vital force theory, suggested earlier by Berzelius.
[3]
References
- ^ MacLean, Elizabeth J.; Harris, Kenneth D. M.; Kariuki, Benson M.; Kitchin, Simon J.; Tykwinski, Rik R.; Swainson, Ian P.; Dunitz; Jack D. (2003). "Ammonium cyanate shows N-H···N hydrogen bonding, not N-H···O". Journal of the American Chemical Society. 125: 14449–14451. doi:10.1021/ja021156x.
- ^ Friedrich Wöhler (1828). "Ueber künstliche Bildung des Harnstoffs". Annalen der Physik und Chemie. 88 (2): 253–256. Bibcode:1828AnP....88..253W. doi:10.1002/andp.18280880206.
- ^ Shorter, J. (1978). "The conversion of ammonium cyanate into urea. A saga on Reaction mechanisms". Chemical Society Reviews. 7: 1–14. doi:10.1039/CS9780700001.