 | |
 | |
| Names | |
|---|---|
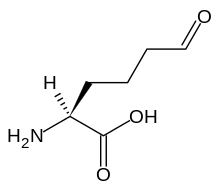
| Preferred IUPAC name (2S)-2-Amino-6-oxohexanoic acid | |
| Other names 2-aminoadipate semialdehyde, 2-amino-5-formylvaleric acid, norvaline, 6-oxo-DL-norleucine | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | allysine |
PubChem CID | |
| UNII | |
| |
SMILES
| |
| Properties | |
| C6H11NO3 | |
| Molar mass | 145.158 g·mol−1 |
| Density | 1.74g/cm3 |
| Boiling point | 295.2 °C (563.4 °F; 568.3 K) |
| Hazards | |
| Flash point | 132.3 °C (270.1 °F; 405.4 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Allysine is a derivative of lysine, used in the production of elastin and collagen. It is produced by the actions of the enzyme lysyl oxidase in the extracellular matrix and is essential in the crosslink formation that stabilizes collagen and elastin.[1]
Allysine in disease
Large quantities of elastin and collagen present in tissue may lead to metastasis: spread of disease. Fibrous tissue containing oxidized collagen may result in a condition known as fibrosis. The oxidation of lysine resides present in collagen creates the aldehyde, aminoadipic-δ-semialdehyde (allysine). Increased allysine concentration in tissues has been correlated to the presence of fibrosis.[2]
Allysine quantification
In one study, allysine is first reacted in acidic conditions (6M HCl, 110 °C, 24 h) with sodium 2-naphthol-7-sulfonate. A fluorescent bis-naphtol allysine is the product. Allysine is then quantified through use of high-performance liquid chromatography (HPLC). The results of this study provide a statistically relevant method in correlating greater concentrations of allysine and fibrotic tissue. The study shows that fibrotic tissue contains 2.5 times more alyssine than non-fibrotic tissue.[3]
See also
References
- ^ Pinnell, S R; Martin, G R (October 1968). "The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone". Proceedings of the National Academy of Sciences of the United States of America. 61 (2): 708–716. Bibcode:1968PNAS...61..708P. doi:10.1073/pnas.61.2.708. ISSN 0027-8424. PMC 225217. PMID 5246001.
- ^ Wahsner, Jessica; Désogère, Pauline; Abston, Eric; Graham-O’Regan, Katherine A.; Wang, Junfeng; Rotile, Nicholas J.; Schirmer, Markus D.; Santos Ferreira, Diêgo Dos; Sui, Jingyi; Fuchs, Bryan C.; Lanuti, Michael (2019-03-25). "68Ga-NODAGA-Indole: An Allysine-Reactive Positron Emission Tomography Probe for Molecular Imaging of Pulmonary Fibrogenesis". Journal of the American Chemical Society. 141 (14): 5593–5596. doi:10.1021/jacs.8b12342. ISSN 0002-7863. PMC 6494104. PMID 30908032.
- ^ Waghorn, Philip A.; Oliveira, Bruno L.; Jones, Chloe M.; Tager, Andrew M.; Caravan, Peter (2017-10-01). "High sensitivity HPLC method for determination of the allysine concentration in tissue by use of a naphthol derivative". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 1064: 7–13. doi:10.1016/j.jchromb.2017.08.032. ISSN 1570-0232. PMC 5662445. PMID 28886479.