 | |
 | |
| Names | |
|---|---|
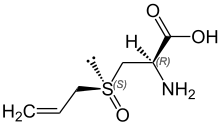
| IUPAC name (2R)-2-amino-3-[(S)-prop-2-enylsulfinyl]propanoic acid | |
| Other names 3-(2-Propenylsulfinyl)alanine (S)-3-(2-Propenylsulfinyl)-L-alanine 3-((S)-Allylsulfinyl)-L-alanine S-Allyl-L-cysteine sulfoxide | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.291 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| UNII | |
| |
SMILES
| |
| Properties | |
| C6H11NO3S | |
| Molar mass | 177.22 g/mol |
| Appearance | White to off white crystalline powder |
| Melting point | 163–165 °C (325–329 °F) |
| Soluble | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements | H315, H319, H335 |
GHS precautionary statements | P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Alliin /ˈæli.ɪn/ is a sulfoxide that is a natural constituent of fresh garlic.[1] It is a derivative of the amino acid cysteine. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic.
Garlic has been used since antiquity as a therapeutic remedy for certain conditions now associated with oxygen toxicity, and, when this was investigated, garlic did indeed show strong antioxidant and hydroxyl radical-scavenging properties, it is presumed owing to the alliin contained within.[2] Alliin has also been found to affect immune responses in blood.[3]
Alliin was the first natural product found to have both carbon- and sulfur-centered stereochemistry.[4]
Chemical synthesis
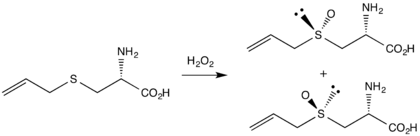
The first reported synthesis, by Stoll and Seebeck in 1951,[5] begins the alkylation of L-cysteine with allyl bromide to form deoxyalliin. Oxidation of this sulfide with hydrogen peroxide gives both diastereomers of L-alliin, differing in the orientation of the oxygen atom on the sulfur stereocenter.
A newer route, reported by Koch and Keusgen in 1998,[6] allows stereospecific oxidation using conditions similar to the Sharpless asymmetric epoxidation. The chiral catalyst is produced from diethyl tartrate and titanium isopropoxide.
References
- ^ Iberl, B; et al. (1990). "Quantitative Determination of Allicin and Alliin from Garlic by HPLC". Planta Med. 56 (3): 320–326. doi:10.1055/s-2006-960969. PMID 17221429.
- ^ Kourounakis, PN; Rekka, EA (November 1991). "Effect on active oxygen species of alliin and Allium sativum (garlic) powder". Res Commun Chem Pathol Pharmacol. 74 (2): 249–252. PMID 1667340.
- ^ Salman, H; et al. (September 1999). "Effect of a garlic derivative (alliin) on peripheral blood cell immune responses". Int J Immunopharmacol. 21 (9): 589–597. doi:10.1016/S0192-0561(99)00038-7. PMID 10501628.
- ^ Eric Block (2009). Garlic and other alliums: the lore and the science. Royal Society of Chemistry. pp. 100–106.
- ^ Stoll , Seeback, Arthur, Ewald (1951). Chemical investigations on alliin, the specific principle of garlic. Advanced Enzymology. Advances in Enzymology - and Related Areas of Molecular Biology. 11. pp. 377–400. doi:10.1002/9780470122563.ch8. ISBN 9780470122563. PMID 24540596.
- ^ Koch, Keusgen (1998). "Diastereoselective synthesis of alliin by an asymmetric sulfur oxidation". Pharmazie. 53 (668–671).