| |
 | |
 | |
| Names | |
|---|---|
| IUPAC name 1,2-Dihydroacenaphthylene | |
| Systematic IUPAC name Tricyclo[6.3.1.04,12]dodeca-1(12),4,6,8,10-pentaene | |
| Other names 1,8-Ethylenenaphthalene peri-Ethylenenaphthalene Naphthyleneethylene Tricyclo[6.3.1.04,12]dodecapentaene | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.336 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3077 |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C12H10 | |
| Molar mass | 154.212 g·mol−1 |
| Appearance | White or pale yellow crystalline powder |
| Density | 1.024 g/cm3 |
| Melting point | 93.4 °C (200.1 °F; 366.5 K) |
| Boiling point | 279 °C (534 °F; 552 K) |
| 0.4 mg/100 ml | |
| Solubility in ethanol | slight |
| Solubility in chloroform | slight |
| Solubility in benzene | very soluble |
| Solubility in acetic acid | soluble |
| -.709·10−6 cm3/g | |
| Hazards | |
| Safety data sheet | ICSC 1674 |
| NFPA 704 (fire diamond) | |
| Flash point | 135 °C (275 °F; 408 K) |
| > 450 °C (842 °F; 723 K) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Acenaphthene is a polycyclic aromatic hydrocarbon (PAH) consisting of naphthalene with an ethylene bridge connecting positions 1 and 8. It is a colourless solid. Coal tar consists of about 0.3% of this compound.[1]
Production and reactions
Acenaphthene was prepared the first time from coal tar by Marcellin Berthelot. Later Berthelot and Bardy synthesized the compound by cyclization of α-ethylnaphthalene. Industrially, it is still obtained from coal tar together with its derivative acenaphthylene (and many other compounds).
Like other arenes, acenaphthene forms complexes with low valent metal centers. One example is (η6-acenaphthene)Mn(CO)3]+.[2]
Uses
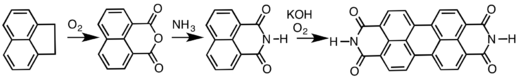
It is used on a large scale to prepare naphthalene dicarboxylic anhydride, which is a precursor to dyes and optical brighteners.[1] Naphthalene dicarboxylic anhydride is the precursor to perylenetetracarboxylic dianhydride, precursor to several commercial pigments and dyes.[3][4]
References
- ^ a b Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter; Höke (2000). "Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227.
- ^ S. B. Kim, S. Lotz, S. Sun, Y. K. Chung, R. D. Pike, D. A. Sweigart "Manganese Tricarbonyl Transfer (MTT) Agents" Inorganic Syntheses, 2010, Vol. 35, 109–128. doi:10.1002/9780470651568.ch6
- ^ K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- ^ Greene, M. "Perylene Pigments" in High Performance Pigments, 2009, Wiley-VCH, Weinheim. pp. 261-274.doi:10.1002/9783527626915.ch16