 | |
 | |
| Names | |
|---|---|
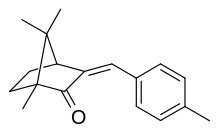
| IUPAC name (3E)-1,7,7-Trimethyl-3-[(4-methylphenyl)methylene]-2-norbornanone | |
| Other names 4-Methylbenzylidene camphor 3-(4-Methylbenzylidene)bornan-2-one 3-(4-Methylbenzylidene)-dl-camphor | |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | 4-MBC |
| ChemSpider | |
| ECHA InfoCard | 100.048.386 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
SMILES
| |
| Properties | |
| C18H22O | |
| Molar mass | 254.37 g/mol |
| Appearance | White crystalline powder |
| Melting point | 66 to 69 °C (151 to 156 °F; 339 to 342 K) |
| Insoluble | |
| Hazards | |
| Main hazards | Xi |
| R-phrases (outdated) | R36/37/38 R50 R53 |
| S-phrases (outdated) | S26 S37/39 S61 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Enzacamene (INN; also known as 4-methylbenzylidene camphor or 4-MBC) is an organic camphor derivative that is used in the cosmetic industry for its ability to protect the skin against UV, specifically UV B radiation. As such, it is used in sunscreen lotions and other skincare products claiming a SPF value. Its tradenames include Eusolex 6300 (Merck) and Parsol 5000 (DSM).
Mechanism
All the camphor-derived sunscreens dissipate the photon energy by cis-trans isomerisation. However, for enzacamene the quantum yield for this isomerization is only between 0.13-0.3. This low quantum yield means that other photochemical processes are also occurring.[2]
Endocrine disruptor
Studies have raised the issue that enzacamene acts as an endocrine disruptor. There is controversy about the estrogenic effects of enzacamene and while one study showed only a relatively minor effect.[3] In addition, there is some evidence that enzacamene may suppress the pituitary-thyroid axis, leading to hypothyroidism.[4]
Approval status
Enzacamene is approved in Canada by Health Canada. It is not approved for use in the United States by the Food and Drug Administration and it is not permitted in Japan nor in Denmark.
See also
References
- ^ 3-(4-METHYLBENZYLIDEN)CAMPHOR at chemicalland21.com
- ^ Sun Protection in Man. Chapter 26: Cantrell, Ann; McGarvey, David J.; Truscott, T. George. Photochemical and photophysical properties of sunscreens.
- ^ Mueller SO; Kling M; Arifin Firzani P; et al. (April 2003). "Activation of estrogen receptor alpha and ERbeta by 4-methylbenzylidene-camphor in human and rat cells: comparison with phyto- and xenoestrogens". Toxicol. Lett. 142 (1–2): 89–101. doi:10.1016/S0378-4274(03)00016-X. PMID 12765243.
- ^ IH Hamann; C Schmutzler; P Kirschmeyer; H Jarry & J Köhrle (2006). "4-Methylbenzylidene-camphor (4MBC) causes pituitary effects comparable to hypothyroidism". Endocrine Abstracts. 11: OC60.