 | |||
| | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name (2,4-Dinitrophenyl)hydrazine | |||
| Other names 2,4-DNPH 2,4-DNP Brady's reagent Borche's reagent | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.918 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
SMILES
| |||
| Properties | |||
| C6H6N4O4 | |||
| Molar mass | 198.14 g/mol | ||
| Appearance | Red or orange powder | ||
| Melting point | 198 to 202 °C (388 to 396 °F; 471 to 475 K) dec. | ||
| Slight | |||
| Hazards | |||
| Main hazards | Flammable, possibly carcinogenic | ||
| Safety data sheet | MSDS | ||
| GHS pictograms |   | ||
| GHS Signal word | Warning | ||
GHS hazard statements | H228, H302, H319 | ||
GHS precautionary statements | P210, P240, P241, P264, P270, P280, P301+312, P305+351+338, P330, P337+313, P370+378, P501 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
2,4-Dinitrophenylhydrazine (DNPH) is the organic compound C6H3(NO2)2NHNH2. Dinitrophenylhydrazine is a red to orange solid. It is a substituted hydrazine. The solid is relatively sensitive to shock and friction. For this reason dinitrophenylhydrazine is usually handled as a wet powder. DNPH is a precursor to the drug Sivifene.
Synthesis
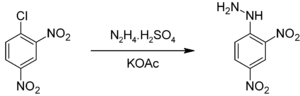
It can be prepared by the reaction of hydrazine sulfate with 2,4-dinitrochlorobenzene:[1]
DNP test
DNPH is a reagent in instructional laboratories on qualitative organic analysis. Brady's reagent or Borche's reagent, is prepared by dissolving 2,4-dinitrophenylhydrazine in a solution containing methanol and some concentrated sulfuric acid. This solution is used to detect ketones and aldehydes. A positive test is signalled by the formation of a yellow, orange or red precipitate of the dinitrophenylhydrazone. Aromatic carbonyls give red precipitates whereas aliphatic carbonyls give more yellow color.[2] The reaction between 2,4-dinitrophenylhydrazine and a generic ketone to form a hydrazone is shown below:
- RR'C=O + C6H3(NO2)2NHNH2 → C6H3(NO2)2NHN=CRR' + H2O
This reaction is, overall, a condensation reaction as two molecules joining together with loss of water. Mechanistically, it is an example of addition-elimination reaction: nucleophilic addition of the -NH2 group to the C=O carbonyl group, followed by the elimination of a H2O molecule:[3]
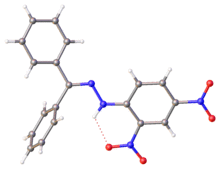 X-ray structure of DNP-derived hydrazone of benzophenone. Selected parameters: C=N, 128 pm; N-N, 1.38 pm, N-N-C(Ar), 119[4]
X-ray structure of DNP-derived hydrazone of benzophenone. Selected parameters: C=N, 128 pm; N-N, 1.38 pm, N-N-C(Ar), 119[4]
DNP-derived hydrazones have characteristic melting points, facilitating identification of the carbonyl. In particular, the use of 2,4-dinitrophenylhydrazine was developed by Brady and Elsmie.[5] Modern spectroscopic and spectrometric techniques have superseded these techniques.
Dinitrophenylhydrazine does not react with other carbonyl-containing functional groups such as carboxylic acids, amides, and esters, for which there is resonance-associated stability as a lone-pair of electrons interacts with the p orbital of the carbonyl carbon resulting in increased delocalization in the molecule. This stability would be lost by addition of a reagent to the carbonyl group. Hence, these compounds are more resistant to addition reactions. Also, with carboxylic acids, there is the effect of the compound acting as a base, leaving the resulting carboxylate negatively charged and hence no longer vulnerable to nucleophilic attack.
Safety
Explosions have resulted from the use of DNPH.[6]
See also
- Tollens' reagent
- Fehling's reagent
- Schiff test
References
- ^ Allen, C. F. H. (1933). "2,4-Dinitrophenylhydrazine". Organic Syntheses. 13: 36. doi:10.15227/orgsyn.013.0036.
- ^ http://wiki.colby.edu/download/attachments/110920618/Experiment+%232.pdf?version=1&modificationDate=1265312071267
- ^ Adapted from Chemistry in Context, 4th Edition, 2000, Graham Hill and John Holman
- ^ Tameem, Abdassalam Abdelhafiz; Salhin, Abdussalam; Saad, Bahruddin; Rahman, Ismail Ab.; Saleh, Muhammad Idiris; Ng, Shea-Lin; Fun, Hoong-Kun (2006). "Benzophenone 2,4-dinitrophenylhydrazone". Acta Crystallographica Section e Structure Reports Online. 62 (12): o5686–o5688. doi:10.1107/S1600536806048112.
- ^ Brady, Oscar L.; Elsmie, Gladys V. (1926). "The use of 2:4-dinitrophenylhydrazine as a reagent for aldehydes and ketones". Analyst. 51 (599): 77–78. Bibcode:1926Ana....51...77B. doi:10.1039/AN9265100077.
- ^ "Bomb disposal squads detonate chemical stocks in British schools". The Guardian. 2 November 2016. Retrieved 19 March 2018.