 | |
 | |
 | |
| Names | |
|---|---|
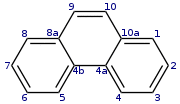
| Preferred IUPAC name Phenanthrene | |
| Other names Tricyclo[8.4.0.02,7]tetradeca-1,3,5,7,9,11,13-heptaene | |
| Identifiers | |
3D model (JSmol) | |
| 1905428 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.437 |
| EC Number |
|
Gmelin Reference | 28699 |
| KEGG | |
| MeSH | C031181 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C14H10 |
| Molar mass | 178.234 g·mol−1 |
| Appearance | Colorless solid |
| Density | 1.18 g/cm3[1] |
| Melting point | 101 °C (214 °F; 374 K)[1] |
| Boiling point | 332 °C (630 °F; 605 K)[1] |
| 1.6 mg/L[1] | |
| -127.9·10−6 cm3/mol | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 171 °C (340 °F; 444 K)[1] |
| Structure | |
| C2v[2] | |
Dipole moment | 0 D |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Phenanthrene is a likely carcinogenic compound that poses a large toxicity risk to exposed living organisms. It is a polycyclic aromatic hydrocarbon (PAH)-which are a large group of organic compounds occurring in groups of two or more. [3] Phenanthrene occurs naturally and also is a man-made chemical. Commonly, humans are exposed to phenanthrene through inhalation of cigarette smoke but there are many routes of exposure. Evidence, proven through animal studies, shows that phenanthrene is a potential carcinogen.[3] It appears as a colorless, crystal-like solid but can also look yellow.[4]
Phenanthrene is used to make dyes, plastics and pesticides, explosives and drugs. It has also been used to make bile acids, cholesterol and steroids.[4]
The compound with a phenanthrene skeleton and nitrogens at the 4 and 5 positions is known as phenanthroline.
Chemistry
Phenanthrene is nearly insoluble in water but is soluble in most low polarity organic solvents such as toluene, carbon tetrachloride, ether, chloroform, acetic acid and benzene.
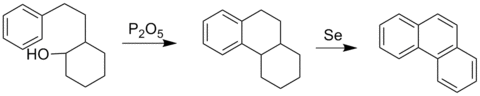
The Bardhan–Sengupta phenanthrene synthesis is a classic way to make phenanthrenes.[5]
This process involves electrophilic aromatic substitution using a tethered cyclohexanol group using diphosphorus pentoxide, which closes the central ring onto an existing aromatic ring. Dehydrogenation using selenium converts the other rings into aromatic ones as well. The aromatization of six-membered rings by selenium is not clearly understood, but it does produce H2Se.
Phenanthrene can also be obtained photochemically from certain diarylethenes.
Reactions of phenanthrene typically occur at the 9 and 10 positions, including:
- Organic oxidation to phenanthrenequinone with chromic acid[6]
- Organic reduction to 9,10-dihydrophenanthrene with hydrogen gas and raney nickel[7]
- Electrophilic halogenation to 9-bromophenanthrene with bromine[8]
- Aromatic sulfonation to 2 and 3-phenanthrenesulfonic acids with sulfuric acid[9]
- Ozonolysis to diphenylaldehyde[10]
Canonical forms
Phenanthrene is more stable than its linear isomer anthracene. A classic and well established explanation is based on Clar's rule. A novel theory invokes so-called stabilizing hydrogen-hydrogen bonds between the C4 and C5 hydrogen atoms.
Natural occurrences
Ravatite is a natural mineral consisting of phenanthrene.[11] It is found in small amounts among a few coal burning sites. Ravatite represents a small group of organic minerals.
In plants
See also
References
- ^ a b c d e Record of CAS RN 85-01-8 in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Peter Atkins, J. D. P., Atkins' Physical Chemistry. Oxford: 2010. Pg.443
- ^ a b "Phenanthrene" (PDF). EPA.gov. Retrieved 3 October 2020.
- ^ a b "Phenanthrene Fact Sheet" (PDF). archive.epa.gov. U.S. Environmental Protection Agency. Retrieved 19 July 2019.
- ^ "Bardhan Sengupta Synthesis". Comprehensive Organic Name Reactions and Reagents. 49. 2010. pp. 215–219. doi:10.1002/9780470638859.conrr049.
- ^ Organic Syntheses, Coll. Vol. 4, p.757 (1963); Vol. 34, p.76 (1954) Link
- ^ Organic Syntheses, Coll. Vol. 4, p.313 (1963); Vol. 34, p.31 (1954) Link.
- ^ Organic Syntheses, Coll. Vol. 3, p.134 (1955); Vol. 28, p.19 (1948) Link.
- ^ Organic Syntheses, Coll. Vol. 2, p.482 (1943); Vol. 16, p.63 (1936) Link.
- ^ Organic Syntheses, Coll. Vol. 5, p.489 (1973); Vol. 41, p.41 (1961) Link.
- ^ Ravatite Mineral Data
External links
- Phenanthrene at scorecard.org