 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.200 |
| Chemical and physical data | |
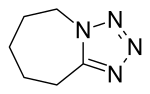
| Formula | C6H10N4 |
| Molar mass | 138.174 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
| |
| (verify) | |
Pentylenetetrazol, also known as pentylenetetrazole, metrazol, pentetrazol (INN), pentamethylenetetrazol, Corazol, Cardiazol, deumacard or PTZ, is a drug formerly used as a circulatory and respiratory stimulant. High doses cause convulsions, as discovered by the Hungarian-American neurologist and psychiatrist Ladislas J. Meduna in 1934. It has been used in convulsive therapy, and was found to be effective—primarily for depression—but side-effects such as uncontrolled seizures were difficult to avoid.[1] In 1939 pentylenetetrazol was replaced by electroconvulsive therapy, easier to administer, as the preferred method for inducing seizures in England's mental hospitals. In the US its approval by the FDA was revoked in 1982.[2] It is used in Italy as a cardio-respiratory stimulant in combination with codeine in a cough suppressant drug.[3]
Mechanism
The mechanism of pentylenetetrazol is not well understood, and it may have multiple mechanisms of action. In 1984, Squires et al. published a report analyzing pentylenetetrazol and several structurally related convulsant drugs. They found that in vivo convulsant potency was strongly correlated to in vitro affinity to the picrotoxin binding site on the GABA-A receptor complex. Many GABA-A ligands are effective anticonvulsants, such as the sedatives diazepam and phenobarbital, but presumably pentylenetetrazol has the opposite effect when it binds to the GABA-A receptor.[4]
Several studies have focused on the way pentylenetetrazol influences neuronal ion channels. A 1987 study found that pentylenetetrazol increases calcium influx and sodium influx, both of which depolarize the neuron. Because these effects were antagonized by calcium channel blockers, it was concluded that pentylenetetrazol acts at calcium channels, and it causes calcium channels to lose selectivity and conduct sodium ions as well.[5]
Research
Pentylenetetrazol has been used experimentally to study seizure phenomena and to identify pharmaceuticals that may control seizure susceptibility. For instance, can model status epilepticus in animal models. Pentylenetetrazol is also a prototypical anxiogenic drug and, has been extensively utilized in animal models of anxiety. Pentylenetetrazol produces a reliable discriminative stimulus which is largely mediated by the GABAA receptor. Several classes of compounds can modulate the pentylenetetrazol discriminative stimulus including 5-HT1A, 5-HT3, NMDA, glycine, and L-type calcium channel ligands.[6]
See also
- List of investigational sleep drugs
- GABAA receptor negative allosteric modulator
- GABAA receptor § Ligands
References
- ^ Read, Charles F. (1940). "Consequences of metrazol shock therapy". American Journal of Psychiatry. 97 (3): 667–76. doi:10.1176/ajp.97.3.667.
- ^ Minkel JR (February 25, 2007). "Drug May Counteract Down Syndrome". Scientific American. Retrieved 2007-03-20.
- ^ "Cardiazol-Paracodina". Agenzia Italiana del Farmaco.
- ^ Squires RF, Saederup E, Crawley JN, Skolnick P, Paul SM (1984). "Convulsant potencies of tetrazoles are highly correlated with actions on GABA / benzodiazepine / picrotoxin receptor complexes in brain". Life Sci. 35 (14): 1439–44. doi:10.1016/0024-3205(84)90159-0. PMID 6090836.
- ^ Papp A, Fehér O, Erdélyi L (1987). "The ionic mechanism of the pentylenetetrazol convulsions". Acta Biol. Hung. 38 (3–4): 349–61. PMID 3503442.
- ^ Jung ME, Lal H, Gatch MB (2002). "The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: recent developments". Neurosci. Biobehav. Rev. 26 (4): 429–39. doi:10.1016/S0149-7634(02)00010-6. PMID 12204190.