 | |
 | |
| Names | |
|---|---|
| IUPAC name 1,3-Bis(2-chloroethyl)-1-nitrosourea[1] | |
| Other names N,N’-Bis(2-chloroethyl)-N-nitrosourea | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.309 |
| EC Number |
|
| KEGG | |
| MeSH | Carmustine |
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2811 |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
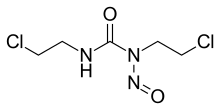
Chemical formula | C5H9Cl2N3O2 |
| Molar mass | 214.05 g·mol−1 |
| Appearance | Orange crystals |
| Odor | Odourless |
| Melting point | 30 °C (86 °F; 303 K) |
| log P | 1.375 |
| Acidity (pKa) | 10.194 |
| Basicity (pKb) | 3.803 |
| Pharmacology | |
| L01AD01 (WHO) | |
| Legal status |
|
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements | H300, H350, H360 |
GHS precautionary statements | P301+310, P308+313 |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 20 mg kg−1 (oral, rat) |
| Related compounds | |
Related ureas | Dimethylurea |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Carmustine (bis-chloroethylnitrosourea, BCNU, BiCNU) is a medication used mainly for chemotherapy. It is a nitrogen mustard β-chloro-nitrosourea compound used as an alkylating agent.
Description
Carmustine is an orange-yellow solid medication used mainly for chemotherapy. It is a nitrogen mustard β-chloro-nitrosourea compound.
Mechanism of action
As an alkylating agent, carmustine can form interstrand crosslinks in DNA, which prevents DNA replication and DNA transcription.
Uses
Carmustine is used as an alkylating agent to treat several types of brain cancer including glioma, glioblastoma multiforme, medulloblastoma and astrocytoma), multiple myeloma, and lymphoma (Hodgkin's and non-Hodgkin).
BCNU is sometimes used in conjunction with alkyl guanine transferase (AGT) inhibitors, such as O6-benzylguanine. The AGT-inhibitors increase the efficacy of carmustine by inhibiting the direct reversal pathway of DNA repair, which will prevent formation of the interstrand crosslink between the N1 of guanine and the N3 of cytosine.
It is also used as part of a chemotherapeutic protocol in preparation for hematological stem cell transplantation, a type of bone marrow transplant, in order to reduce the white blood cell count in the recipient.[2] Use under this protocol, usually with fludarabine and melphalan, was developed by oncologists at the University of Texas MD Anderson Cancer Center.
Implants
In the treatment of brain tumours, the U.S. Food and Drug Administration (FDA) approved biodegradable discs infused with carmustine (Gliadel).[3] They are implanted under the skull during a surgery called a craniotomy. The disc allows for controlled release of carmustine in the extracellular fluid of the brain, thus eliminating the need for the encapsulated drug to cross the blood-brain barrier.[4]
Production
Carmustine for injection was marketed under the name BiCNU by Bristol-Myers Squibb[5] and now by Emcure Pharmaceuticals.[6] In India it is sold under various brand names, including Consium.. The product is now available as a generic version with other manufacturers offering the product licensed in the US and EU markets.
See also
- Bendamustine
- Lomustine
- Semustine
References
- ^ CID 2578 from PubChem
- ^ Damaj, G; Cornillon, J; Bouabdallah, K; Gressin, R; Vigouroux, S; Gastinne, T; Ranchon, F; Ghésquières, H; Salles, G; Yakoub-Agha, I; Gyan, E (2017). "Carmustine replacement in intensive chemotherapy preceding reinjection of autologous HSCs in Hodgkin and non-Hodgkin lymphoma: A review". Bone Marrow Transplantation. 52 (7): 941–949. doi:10.1038/bmt.2016.340. PMID 28112752.
- ^ Ewend MG, Brem S, Gilbert M, et al. (June 2007). "Treatment of single brain metastasis with resection, intracavity carmustine polymer wafers, and radiation therapy is safe and provides excellent local control". Clin. Cancer Res. 13 (12): 3637–41. doi:10.1158/1078-0432.CCR-06-2095. PMID 17575228.
- ^ "Hopkins Medicine Magazine - In Spite of All Odds". Archived from the original on 2014-11-20. Retrieved 2014-07-08.
- ^ "Archived copy". Archived from the original on 2014-07-11. Retrieved 2015-01-31.
- ^ "Emcure Press release" (PDF). Archived from the original (PDF) on 2014-07-02. Retrieved 2015-01-31.