 | |
| Names | |
|---|---|
| IUPAC name Tin(IV) sulfide | |
| Other names Tin disulfide, Stannic sulfide, Mosaic gold | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ECHA InfoCard | 100.013.867 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | S2Sn |
| Molar mass | 182.83 g·mol−1 |
| Appearance | Gold-yellow powder |
| Odor | Odorless |
| Density | 4.5 g/cm3[1] |
| Melting point | 600 °C (1,112 °F; 873 K) decomposes[1] |
| Insoluble | |
| Solubility | Soluble in aq. alkalis, decompose in aqua regia[1] Insoluble in alkyl acetates, acetone[2] |
| Structure | |
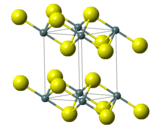
Crystal structure | Rhombohedral, hP3[3] |
| P3m1, No. 164[3] | |
| 3 2/m[3] | |
a = 3.65 Å, c = 5.88 Å[3] α = 90°, β = 90°, γ = 120° | |
| Octahedral (Sn4+)[3] | |
| Hazards | |
| GHS pictograms |  [4] [4] |
| GHS Signal word | Warning |
GHS hazard statements | H302, H312, H315, H319, H332, H335[4] |
GHS precautionary statements | P261, P280, P301+312, P302+352, P304+340, P305+351+338, P332+313[4] |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tin(IV) sulfide is a compound with the formula SnS
2. The compound crystallizes in the cadmium iodide motif, with the Sn(IV) situated in "octahedral holes' defined by six sulfide centers.[5] It occurs naturally as the rare mineral berndtite.[6] It is useful as semiconductor material with band gap 2.2 eV.[7]
Reactions
The compound precipitates as a brown solid upon the addition of H
2S to solutions of tin(IV) species. This reaction is reversed at low pH. Crystalline SnS
2 has a bronze color and is used in decorative coating[8] where it is known as mosaic gold.
The material also reacts with sulfide salts to give a series of thiostannates with the formula [SnS
2]
m[S]2n−
n. A simplified equation for this depolymerization reaction is
- SnS
2 + S2−
→ 1/x[SnS2−
3]
x.
References
- ^ a b c Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Comey, Arthur Messinger; Hahn, Dorothy A. (February 1921). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York: The MacMillan Company. p. 1080.
- ^ a b c d e Voort, G.F. Vander, ed. (2004). "Crystal Structure*" (PDF). ASM Handbook. 9 (Metallography and Microstructures): 29–43. doi:10.1361/asmhba0003722 (inactive 2021-01-11).
- ^ a b c d "SDS of Stannic sulfide" (PDF). https://www.pfaltzandbauer.com. Connecticut, USA: Pfaltz & Bauer, Inc. Retrieved 2014-07-13.
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Vaughan, D. J.; Craig, J. R. "Mineral Chemistry of Metal Sulfides" Cambridge University Press, Cambridge: 1978. ISBN 0-521-21489-0.
- ^ L.A.Burton et al., J. Mater. Chem. A, 2016, 4, 1312-1318 DOI: 10.1039/C5TA08214E.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
External links
