 | |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
| ECHA InfoCard | 100.031.277 |
PubChem CID | |
| Properties | |
Chemical formula | Ag2Te |
| Molar mass | 341.3364 g/mol |
| Appearance | grey-black crystals |
| Density | 8.318 g/cm³ |
| Melting point | 955 °C (1,751 °F; 1,228 K) |
Refractive index (nD) | 3.4 |
| Structure | |
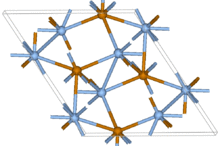
Crystal structure | Monoclinic, mP12 |
| P21/c, No. 14 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Silver telluride (Ag2Te) is a chemical compound, a telluride of silver, also known as disilver telluride or silver(I) telluride. It forms a monoclinic crystal. In a wider sense, silver telluride can be used to denote AgTe (silver(II) telluride, a metastable compound) or Ag5Te3.
Silver(I) telluride occurs naturally as the mineral hessite, whereas silver(II) telluride is known as empressite.
Silver telluride is a semiconductor which can be doped both n-type and p-type. Stoichiometric Ag2Te has n-type conductivity. On heating silver is lost from the material.
Non-stoichiometric silver telluride has shown extraordinary magnetoresistance.
References
- Aliev, F. F. (2002). "Phase Transition of Ag_Enriched Ag2Te". Inorganic Materials. 38 (10): 995. doi:10.1023/A:1020512918319.
- Chuprakov, I. S.; Dahmen, K. H. (1998). "Large positive magnetoresistance in thin films of silver telluride". Applied Physics Letters. 72 (17): 2165. Bibcode:1998ApPhL..72.2165C. doi:10.1063/1.121309.
- Dalven, Richard (1966). "Fundamental Optical Absorption in β-Silver Telluride". Physical Review Letters. 16 (8): 311. Bibcode:1966PhRvL..16..311D. doi:10.1103/PhysRevLett.16.311.
See also
Related materials
- Silver selenide
- Silver sulfide