 | |
amide_unsolvated_from_crystal.gif) | |
| Names | |
|---|---|
| Preferred IUPAC name Potassium 1,1,1-trimethyl-N-(trimethylsilyl)silanaminide | |
| Other names Potassium hexamethyldisilazide | |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | KHMDS |
| ChemSpider | |
| ECHA InfoCard | 100.115.605 |
PubChem CID | |
| UN number | 3263 |
CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | KSi 2C 6NH 18 |
| Molar mass | 199.4831 g mol−1 |
| Appearance | White, opaque crystals |
| Reacts | |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
GHS hazard statements | H314[1] |
GHS precautionary statements | P280, P305+351+338, P310[1] |
| Related compounds | |
Other cations | Lithium bis(trimethylsilyl)amide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Potassium bis(trimethylsilyl)amide (commonly abbreviated as KHMDS, Potassium(K) HexaMethylDiSilazide) is the chemical compound with the formula ((CH3)3Si)2NK. It is a strong, non-nucleophilic base with an approximate pKa of 26 (compare to lithium diisopropylamide, at 36).
Structure
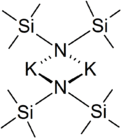
In the solid state, the unsolvated compound is dimeric, with two potassium and two nitrogen atoms forming a square. This compound is soluble in hydrocarbon solvents and conducts electricity poorly in solution and in the melt. This is attributed to very strong ion pairing.[2]
See also
- Metal bis(trimethylsilyl)amides
References
- ^ a b Potassium bis(trimethylsilyl)amide, Sigma-Aldrich
- ^ Tesh, Kris F.; Hanusa, Timothy P.; Huffman, John C. (1990). "Ion pairing in [bis(trimethylsilyl)amido]potassium: The x-ray crystal structure of unsolvated [KN(SiMe3)2]2". Inorg. Chem. 29 (8): 1584–1586. doi:10.1021/ic00333a029.